Summary
Multiple mechanisms that contribute to cardiovascular disease in patients with diabetes mellitus have been identified. Diabetic neuropathy can manifest as cardiac autonomic neuropathy and directly affect heart function. In addition, obstructive sleep apnea is associated with cardiovascular events and death. Hypoglycemia episodes can cause cardiac ischemia, atherothrombosis, and endothelial dysfunction.
- diabetes mellitus
- cardiovascular disease
- obstructive sleep apnea
- diabetic neuropathy
- cardiac autonomic neuropathy
- cardiometabolic disorder
- hyperglycemia/hypoglycemia
Over the last 20 years, the prevalence of diabetes mellitus (DM) in the United States has nearly doubled to 9.3% [Selvin E et al. Ann Intern Med. 2014], with rising prevalence observed in children and adolescents [Dabelea D et al. JAMA. 2014]. Rodica Pop-Busui, MD, PhD, University of Michigan, Ann Arbor, Michigan, USA, discussed the role of diabetic neuropathy on cardiovascular (CV) risk in patients with DM.
Neuropathies affect up to 50% of patients with DM [Pop-Busui R et al. Diabetes Care. 2013]. The diagnosis of peripheral diabetic neuropathy can be aided by using a simple clinical tool, the Michigan Neuropathy Screening Instrument [Herman WH et al. Diabet Med. 2012; Feldman et al. Diabetes Care. 1994]. In addition, the type of symptoms that a patient experiences can be indicative of the type of nerve fibers that are affected (Table 1). Importantly, the heart is innervated by both sympathetic and parasympathetic nerves.
Symptoms Indicating Nerve Fiber–Type Involvement
Diabetic neuropathy is associated with high morbidity and low quality of life and is an independent predictor of mortality in patients with type 1 DM (T1DM) and type 2 DM (T2DM). For instance, the EURODIAB IDDM Prospective Complications Study [Soedamah-Muthu SS et al. Diabetes Care. 2008] reported that both CV autonomic and peripheral neuropathy were associated with similar CV disease (CVD) mortality risk after 7 years of follow-up. An analysis of > 8000 patients with T2DM enrolled in the ACCORD trial [Pop-Busui R et al. Diabetes Care. 2010] who had valid cardiac autonomic neuropathy (CAN) data found that presence of CAN at baseline was associated with higher all-cause and CVD mortality risk after 3.5 years of follow-up.
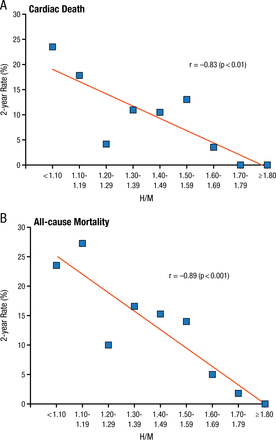
Similarly, a post hoc analysis of the ACCORD trial found that all-cause mortality was significantly associated with self-reported history of neuropathy (P Interaction = .0008) [Calles-Escadrón J et al. Diabetes Care. 2010]. Furthermore, the ADMIRE-HF trial [Jacobson AF et al. J Am Coll Cardiol. 2010] demonstrated that both cardiac death (r = –0.83; P < .01) and all-cause mortality (r = –0.89; P < .001) were highly correlated with lower iodine-123 metaiodobenzylguanidine retention at baseline, an imaging technique to assess integrity of cardiac sympathetic innervation and CAN (Figure 1).
Cardiac Autonomic Neuropathy as Measured by mIBG in the ADMIRE-HF Trial
H/M, heart to mediastinum; mIBG, metaiodobenzylguanidine.
Reprinted from J Am Coll Cardiol, 2010;55:2212-2221, Jacobson AF et al, Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. Copyright with permission from American College of Cardiology Foundation.
Emerging data suggest a link among CAN, left ventricle remodeling, and myocardial dysfunction as reported by several studies. The DCCT/EDIC study [Pop-Busui R et al. J Am Coll Cardiol. 2013] found that patients with DM and CAN had significantly greater cardiac output (P < .0001), left ventricular mass (P < .0001), and mass-to-volume ratio (P < .0001), compared with patients who did not have CAN.
Abd A. Tahrani, MD, PhD, University of Birmingham, Birmingham, United Kingdom, discussed the possible link between obstructive sleep apnea (OSA) and CVD.
OSA has been linked to many factors that are involved in the pathogenesis of CVD risk in population studies, such as hypertension [Peppard PE et al. N Engl J Med. 2000], insulin resistance [Tahrani AA et al. Curr Opin Pulm Med. 2013], inflammation [Lavie L. Front Biosci (Elite Ed). 2012], and obesity.
Continuous positive airway pressure (CPAP) has been shown to lower blood pressure (BP) and improve insulin resistance in randomized controlled trials [Hu X et al. J Clin Hypertens (Greenwich). 2015; Iftikhar IH et al. J Clin Sleep Med. 2013]. Limited data also suggest that CPAP can improve insulin resistance and BP in patients with T2DM [Chen L et al. Arch Med Sci. 2014; Myhill PC et al. J Clin Endocrinol Metab. 2012].
OSA has been more directly linked to CVD. In one study, the cumulative incidence of fatal and nonfatal CV events over an average follow-up of 10.1 years was higher in patients with untreated severe OSA compared with CPAP-treated patients, patients with mild OSA, simple snorers, and the control group [Marin JM et al. Lancet. 2005]. In patients with T2DM, OSA was significantly associated with stroke (OR, 2.57; 95% CI, 1.03 to 6.42; P = .04) in a cross-sectional analysis of a subgroup of the Look AHEAD trial [Rice TB et al. Sleep. 2012].
OSA has also been shown to be associated with increased oxidative and nitrosative stress and microvascular disease in patients with T2DM [Tahrani AA et al. Am J Respir Crit Care Med. 2012], as well as peripheral neuropathy (60% vs 30%), nephropathy (49% vs 24%), and retinopathy in patients with T2DM in cross-sectional studies [Tahrani AA et al. Diabetes Care. 2013; Tahrani AA et al. Am J Respir Crit Care Med. 2012; West SD et al. Diabet Med. 2010]. A recent study also found that patients with T1DM and CAN had a greater prevalence of OSA compared with patients who did not have CAN (67% vs 23%; P = .02) [Janovsky CC et al. Front Endocrinol (Lausanne). 2014].
Furthermore, patients with mild and moderate-to-severe OSA had greater reduction in estimated glomerular filtration rate compared to those without OSA in patients with T2DM over 2.5 years of follow-up [Tahrani AA et al. Diabetes Care. 2013]. Current prospective studies and randomized controlled trials are ongoing to assess the relationship between OSA and diabetic microvascular complications and the impact of CPAP in patients with T2DM.
Stephen Neil Davis, MBBS, University of Maryland School of Medicine, Baltimore, Maryland, USA, discussed hypoglycemia and its role in severe adverse cardiac events. Multiple studies have demonstrated that hypoglycemia is associated with an increased risk of CV events and all-cause mortality [Khunti K et al. Diabetes Care. 2015].
A potential mechanism for this phenomenon is cardiac ischemia, which is associated with hypoglycemic episodes. In a 2003 study of 19 patients with T2DM, 10 out of 54 episodes of hypoglycemia occurred with chest pain, and of these 10 episodes, 4 were associated with significant echocardiograph abnormalities (P < .01).
Other potential mechanisms are atherothrombosis and endothelial dysfunction. A recent study found that acute and repeated hypoglycemia resulted in increased levels of markers of atherothrombosis, including vascular cell adhesion protein 1, P-selectin, interleukin 6, and TNF-α [Joy NG et al. Diabetes. 2015]. In addition, hypoglycemia resulted in increased levels of plasminogen activator inhibitor 1 and thrombin-antithrombin complex, as well as decreased flow-mediated dilation via endogenous and exogenous nitric oxide–mediated vasodilation.
Several agents are under investigation to determine their role in the counterregulatory response during hypoglycemia, including fructose [Gabriely I, Shamoon H. Diabetes. 2005], naloxone [Vele S et al. J Clin Endocrinol Metab. 2011], dehydroepiandrostenedione, and fluoxetine [Briscoe VJ et al. Diabetes. 2008].
In conclusion, there are multiple mechanisms that are attributed to CVD in patients with DM. In a direct role, neuropathy can lead to CAN and directly affect heart function. In an indirect role, patients with DM and OSA are at an increased risk of CV events and CV death. In addition, patients with OSA are more likely to experience diabetic neuropathy compared with patients who do not have OSA. Finally, hypoglycemic episodes can cause cardiac ischemia and lead to atherothrombosis and endothelial dysfunction.
- © 2015 SAGE Publications