Summary
The 2 currently available categories of osteoporosis therapies are the antiresorptive agents, which decrease bone resorption and bone formation, and parathyroid hormone agonists, which increase bone formation and bone resorption. Cathepsin K inhibition is a new mechanism of action that inhibits bone resorption while preserving bone formation.
- anabolic
- bone formation
- bone remodeling
- bone resorption
- cathepsin K
- osteoporosis
- parathyroid hormone
- parathyroid hormone-related protein
- postmenopausal
- RANKL
- sclerostin
- hormone therapy
The parathyroid hormone (1-34) [PTH(1-34)], teriparatide, is the only anabolic agent approved in the United States. PTH(1-34) stimulates bone formation, but it also stimulates bone resorption, limiting its anabolic effect. Teriparatide reduces fractures and increases bone mineral density (BMD) but is associated with hypercalcemia and hypercalciuria.
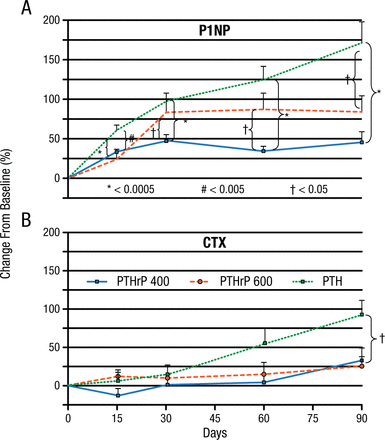
According to Mara J. Horwitz, MD, University of Pittsburgh, Pittsburgh, Pennsylvania, USA, in phase 1 studies, parathyroid hormone–related protein (1-36) [PTHrP(1-36)] increased markers of bone formation but had little effect on bone resorption and did not cause hypercalcemia. The phase 2 PrOP study randomized 105 postmenopausal women to PTHrP(1-36), 400 mg/d; PTHrP(1-36), 600 mg/d; or PTH(1-34), 20 mg/d [Horwitz MJ et al. J Bone Miner Res. 2013]. There was a very early significant increase in aminoterminal propeptide of procollagen 1 (P1NP), a marker of bone formation, in all 3 groups, but by day 90, the increase in the PTH(1-34) group was significantly greater than in the PTHrP(1-36) groups. The increase in the bone resorption marker carboxyterminal telopeptides of collagen 1 (CTX) was delayed, with an increase in the PTH(1-34) group at day 60 and the PTHrP(1-36) groups at day 90 (Figure 1).
PrOP Study: Change From Baseline in P1NP and CTX
CTX, carboxy-terminal telopeptides of collagen 1; P1NP, amino-terminal telopeptides of procollagen 1.
*P < .0005
#P < .005
†P < .05
Reprinted from J Bone Miner Res, Horowitz MJ et al, A comparison of parathyroid hormone-related protein (1-36) and parathyroid hormone (1-34) on markers of bone turnover and bone density in postmenopausal women: The PrOP study, 2013;28(11):2266-2276, Copyright © with permission from American Society for Bone and Mineral Research.
Lumbar spine (LS) BMD increased significantly in all 3 groups (P < .05). The increase in total hip (TH) and femoral neck (FN) BMD was equivalent in each group but was significant only in both PTHrP(1-36) groups for TH (P < .05) and in the PTHrP(1-36)–400 mg group for the FN (all P < .05). On day 90, all groups had the same basal total serum calcium and a small but significant total serum calcium increase 3 to 6 hours postdose (P < .0005), with no difference between the groups. Hypercalcemia occurred in 10 patients receiving PTHrP(1-36)–400 mg, in 8 receiving PTHrP(1-36)–600 mg, and in none receiving PTH(1-34). This study showed that PTHrP(1-36) and PTH(1-34) stimulate bone formation (P1NP) by day 15, with a later and less robust increase in bone resorption (CTX) compared to PTH(1-34).
Abaloparatide, a synthetic peptide analog of PTHrP(1-34), has anabolic activity with less of an increase in bone resorption than PTH(1-34) and less calcium-mobilizing potential. Results of 2 important abaloparatide trials are summarized in Table 1.
Studies of Abaloparatide in Postmenopausal Women With Osteoporosis
PTHrP(1-36) and abaloparatide both have anabolic effects on the LS and TH. Both agents are comparable to teriparatide for increasing LS, FN, and TH bone density. Further testing of PTHrP(1-36) is needed to determine the ideal dosage and its clinical benefits. Abaloparatide led to significant reductions in vertebral and nonvertebral fractures that were at least equivalent to teriparatide, with smaller changes in bone turnover. At 80 mg/d, abaloparatide appears to be safe and well tolerated.
Jacques P. Brown, MD, Laval University, Quebec City, Quebec, Canada, reviewed the available data for romosozumab and blosozumab, humanized monoclonal sclerostin antibodies. Sclerostin is a glycoprotein produced in osteocytes that inhibits bone formation.
Romosozumab is an IgG2 monoclonal antibody with a high affinity for human sclerostin. In animal studies, romosozumab increased bone formation, BMD, and bone strength [Li X et al. J Bone Miner Res. 2010]. Blosozumab is a humanized IgA antisclerostin monoclonal antibody that has neutralized sclerostin activity in animal studies and a high affinity for human sclerostin. In preclinical studies, blosozumab promoted bone formation and improved bone structure and strength. Phase 1 and 2 trials of romosozumab and blosozumab are summarized in Table 2. In these studies, both romosozumab and blosozumab improved BMD in postmenopausal women with low BMD.
Studies of Antisclerostin Monoclonal Antibodies in Postmenopausal Women
A newer antisclerostin monoclonal antibody, BPS804, was evaluated in a 9-month multiple-dose study in postmenopausal women with low BMD. The study was completed in October 2013, but no results have been published.
In summary, the high-affinity humanized sclerostin monoclonal antibodies are currently under evaluation as bone anabolic agents for the treatment of osteoporosis in postmenopausal women. The preliminary results of surrogate end points such as BMD and bone markers are promising and associated with a good safety profile. Antifracture efficacy data should be available soon for romosozumab. Further studies are needed to integrate this new treatment option within current osteoporosis therapies.
Michael R. McClung, MD, Oregon Osteoporosis Center, Portland, Oregon, USA, focused on the osteoclast-osteoblast coupling mechanism to demonstrate the different effects of receptor-activated nuclear factor κB ligand (RANKL) and cathepsin K inhibition on bone remodeling. Coupled bone remodeling involves communication between osteoblasts and osteoclasts via the RANKL pathway [Sims NA, Martin TJ. Bonekey Rep. 2014]. RANKL is a growth-promoting factor secreted by osteoblasts that binds to its receptor, RANK, on preosteoclasts, resulting in differentiation and proliferation of osteoclasts. Osteoclasts modulate osteoblast activity by releasing regulatory factors from bone matrix during bone resorption. The RANKL inhibitor, denosumab, causes a marked decrease in osteoclasts, resulting in reduced bone resorption, followed by a substantial decrease in bone formation.
Cathepsin K, a specialized enzyme highly expressed in osteoclasts, is the main proteolytic enzyme that degrades proteins in bone matrix. Cathepsin K inhibition results in decreased bone resorption but osteoclast number and function are not decreased and may increase, resulting in no change in or increased bone formation. Odanacatib is a highly selective, reversible, and potent inhibitor of cathepsin K [Duong LT. Bonekey Rep. 2012; Leung P et al. Bone. 2011]. In a phase 2 study, odanacatib administered for 5 years to postmenopausal women with low BMD reduced markers of bone resorption, while bone formation markers fell at the outset of treatments but returned to baseline after 2 years. As a consequence, progressive increases in spine and hip BMD were observed [Langdahl B et al. J Bone Miner Res. 2012].
The just-completed phase 3 LOFT trial [McClung MR et al. ASBMR 2014 (abstr 1147)] of odanacatib in 16 000 postmenopausal women with osteoporosis was stopped at the first interim analysis because of robust efficacy. The trial design has been published [Bone HG et al. Osteoporos Int. 2015], but unpublished results show that over about 3 years, odanacatib reduced vertebral fractures by 54%, hip fractures by 47%, and clinical vertebral fractures by 72%. The longer patients were on therapy, the greater the apparent reduction in nonvertebral fracture risk.
Table 3 compares the effects of RANKL (denosumab) and cathepsin K (odanacatib) inhibition.
Comparison of Effects of RANKL Inhibition and Cathepsin K Inhibition
Cathepsin K inhibition is a potential new therapy for osteoporosis with a unique mechanism of action that inhibits bone resorption but preserves osteoclast function and bone formation. Dr McClung concluded that odanacatib promises to be a useful and important addition to treatment options for osteoporosis.
- © 2015 SAGE Publications