Summary
Checkpoint inhibitors have clear evidence of activity against programmed cell death and programmed death ligand in non–small cell lung cancer. Immunotherapies are increasingly being investigated in combinations, though strong immune responses warrant caution. Current therapeutic vaccines have had limited success in lung cancer. Clinical trial strategies are developing to best test targeted therapies.
- nivolumab
- MAGRIT
- lung neoplasms/therapy
- cancer vaccines
- ipilimumab
- pembrolizumab
- MPLD3280A
- MEDI4736
- PD-1 inhibition
- PD-L1 inhibition
- immune checkpoint modulator
- immunotherapies
- clinical trial design
Promising immunotherapies are in development for lung cancer. Many combinations with these drugs are being investigated, though caution on provoking the immune system is needed. Anticancer vaccines have had limited success for lung cancer. Finally, clinical trials are evolving as therapies rapidly change.
Checkpoint Inhibitors
Lung tumors and melanomas display many more mutations than average and have about 200 nonsynonymous mutations per tumor, explained Solange Peters, MD, PhD, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland. In the clinic, immune checkpoint inhibitors target programmed cell death (PD-1), programmed death ligand (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), which all help cancer to avoid immune destruction. Most PD-1 or PD-L1 inhibitors have reached late-phase development.
Across studies, said Prof Peters, the response rate to anti-PD-1/PD-L1 inhibitors in unselected patients is 12% to 25%, though benefit is probably better translated by overall survival (OS) data. Checkpoint responses in non–small cell lung cancer (NSCLC) include unconventional, “immune-related” responses in not more than 5% of patients, with persistent reduction in target lesions or regression following initial progression, which results in the applicability of the usual RECIST criteria in this setting. The safety profile of checkpoint inhibitors is manageable, and > 3 years of follow-up data on nivolumab have found no new safety signals.
A phase 3 trial of nivolumab in advanced, squamous cell NSCLC was recently stopped early due to superior OS with nivolumab treatment (median OS, 9.2 months; 95% CI, 7.3 to 13.3) vs docetaxel (median OS, 6.0 months; 95% CI, 5.1 to 7.3; P = .00025) [Opdivo (package insert). Princeton, NJ: Bristol-Myers Squibb Company; 2015]. A pooled analysis of pembrolizumab used as first- and subsequent-line monotherapy found that tumors shrank for 58% of patients, according to RECIST central review [Garon EB et al. ESMO 2014 (abstr LBA43)]. The response was lasting in patients for whom pembrolizumab had activity. Data are also emerging for the PD-L1 inhibitors MPLD3280A and MEDI4736.
Predicting responsive subgroups is difficult. Smokers respond more than nonsmokers, but never-smokers also respond, including EGFR-mutated, partial-response, anaplastic lymphoma kinase–rearranged tumors. Level of PD-L1 expression has not had a clear association with response rate, progression-free survival (PFS), or OS. PD-L1 is not a reliable biomarker to date, and its evaluation still needs to be refined. Its expression is dynamic not only on tumor cells but also in immune cells, and it is evaluated differently in different trials.
Combination Strategies
While the old misperception was that chemotherapy did not interact with the immune system, the current goal is to increase survival through combinations and sequencing of immunotherapy, chemotherapy, and other therapies, said Martin Reck, MD, PhD, Lung Clinic Grosshansdorf, Grosshansdorf, Germany. However, safety is always a concern. Many chemotherapy and immunotherapy combinations are now in phase 3 trials.
Combining PD-1 inhibition with an EGFR–tyrosine kinase inhibitor may be an option for patients without the T790M mutation in EGFR, but more data are needed.
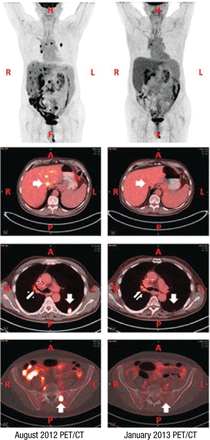
Radiation therapy may increase PD-L1 expression and prime response to immunotherapy. The abscopal effect was reported in a treatment-refractory lung cancer patient who received radiation therapy and ipilimumab, resulting in his tumor shrinking (Figure 1) [Golden EB et al. Cancer Immunol Res. 2013].
Abscopal, or Away From Target, Effect With Radiation Therapy
A, anterior side; CT, computed tomography; F, feet; H, head; L, left side; P, posterior side; PET, positron emission tomography; R, right side.
Reprinted from Cancer Immunol Res, Copyright (2013), Vol 1, Pages 365-372, Golden EB et al, An Abscopal Response to Radiation and Ipilimumab in a Patient with Metastatic Non-Small Cell Lung Cancer, with permission from AACR.
Combining immunotherapies must be done with caution, however, as multiple studies have reported high rates of adverse events and treatment discontinuation.
Vaccines in Lung Cancer
Lung cancer has a strong immunosuppressive environment that has historically led to disappointing immunotherapy results, stated Johan Vansteenkiste, MD, PhD, University Hospital KU Leuven, Leuven Lung Cancer Group, Leuven, Belgium. Recent cancer vaccination studies have had better-defined antigens and adjuvants, low toxicity that defines a unique treatment opportunity, and strong phase 3 data. The L-BLP25 vaccine had a 10-month improvement in OS for the subgroup that received previous concurrent chemoradiation therapy vs placebo (30.8 vs 20.6 months; HR, 0.78; 95% CI, 0.64 to 0.95; P = .016) for stage 3 NSCLC [Butts C et al. Lancet Oncol. 2014].
MAGRIT was the largest therapeutic trial ever done in NSCLC (n = 2272) [NCT00480025]. The vaccination occurred in the adjuvant setting, seeking to eliminate minimal remaining tumor cells after surgery. Treatment with the adjuvant MAGE-A3 did not increase disease-free survival compared with placebo. Though MAGE-A3 led to antigen-specific antibodies and cytotoxic cells, these did not affect patient outcomes. MAGRIT found that therapeutic vaccination with current technologies does not work in lung cancer.
Design of Clinical Trials and Perspectives
The history of oncology is full of failed phase 3 trials, stated David P. Carbone, MD, PhD, Ohio State University, Columbus, Ohio, USA. Now therapeutics are developed in science-based ways with rationally designed drugs and combinations going after defined targets, allowing a priori patient selection strategies based on identifying targets. Biologics have a flatter dose–effect curve. New treatments are improving efficacy, and the bar is rising, with hazard ratios of 0.7 or better being the norm.
Phase 1 trials now use a limited number of doses, drug combinations, and selected populations of patients, with expansion cohorts to find signals. Phase 2 trials use randomized designs, and phase 3 trials are becoming smaller and smarter. Novel clinical trials are using adaptive, Bayesian designs; basket designs that examine multiple diseases for a given marker; and umbrella designs that examine multiple markers for a given disease.
Dr Carbone stated that OS is the best clinical end point, while he feels that PFS is a compromise. Survival benefits should be shown by truly effective therapies, which should provide optimum benefits when used as first-line treatment.
Biomarkers that appear theoretically correct are not always correct, and researchers must consider that populations that are negative for a biomarker are not homogeneous just because they lack the biomarker of interest. Trials to evaluate biomarkers should avoid taking response rate and PFS too seriously.
Targeted therapies can drive targeted escape mechanisms, such as T790M in EGFR. Heterogeneity can exist, even within the tumor of an individual patient. Targeted therapies may have unexpected effects, as inhibiting one pathway may activate another.
New trial designs need to account for the “pseudo-progression” that can occur initially with immunotherapies. Survival benefit should be detected in the absence of response. Trial designs should account for manageable heterogeneity within a single patient, such as brain metastases and single-site progressions. Optimal sequencing should be defined by trial designs, through the use of sequenced or newest-first targeted therapies.
Long-term survival is being improved by modern therapies, but selecting the right patient for the right therapy is a crucial goal. While randomized strategies are still important, allowing patients to cross over both reflects reality and is more ethical.
- © 2015 SAGE Publications