Summary
Next-generation sequencing—identifying new molecular targets in non–small cell lung cancer and subsets of patients who are responsive or resistant to novel compounds—will lead to patient-based therapy, the next step from current target-based treatment. The design of clinical trials is evolving to more efficiently test new compounds for new molecular targets and simultaneously develop predictive biomarkers.
- adenocarcinoma lung cancer
- Biomarkers France study
- non–small cell lung cancer
- squamous cell lung cancer
- next-generation sequencing
- Lung-MAP trial
- genomics
- oncology clinical trials
The emergence of molecular targets identified by genotyping tumors in patients with non–small cell lung cancer (NSCLC) has led to the development of new drugs to manage NSCLC. Next-generation sequencing (NGS) is now being used to identify subsets of patients who are sensitive or resistant to specific drugs and to identify new therapeutic targets that can be tested in clinical trials with novel compounds. This transition from pathologic-guided treatment of NSCLC to target-based treatment will be followed by patient-based therapy specific to genetic alterations identified by NGS. A new paradigm for clinical trials of targeted therapies that includes testing of new predictive biomarkers was reviewed in this session, along with clinical research in France and Germany that has employed target-based treatment.
Bioguided Treatment May Improve Outcomes
A network of genetic centers was established in France by its National Cancer Institute for the routine analysis in daily practice of targetable oncogenes and biomarkers using NGS and to conduct clinical trials, stated Fabrice Barlesi, MD, Aix Marseille University, Marseille, France. The Biomarkers France study in patients with advanced NSCLC was conducted to determine whether it was possible to match the drug to the oncogenic driver for so-called bioguided treatment that is patient specific and improves outcomes.
This study prospectively collected into a centralized database nearly 19 400 molecular analyses and the epidemiologic, clinical, and therapeutic data from the 17 664 corresponding patients. The preliminary findings from 10 000 biomarker analyses were previously reported [Barlesi F et al. ASCO 2013 (abstr 8000)]. The final results have been submitted for publication and could not be presented.
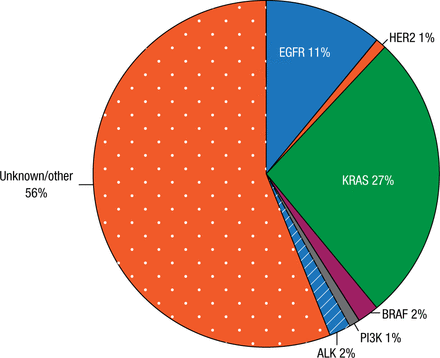
An indication of the final results for the Biomarkers France study may come from a local study conducted at the same time by Prof Barlesi and colleagues in their hospital and a study conducted in the United States. However, the latter 2 studies were limited to adenocarcinoma (ADC), while the Biomarkers France study is not. The local study included 262 patients with stage IV ADC of the lung diagnosed between 2012 and 2013; the genetic profile is shown in Figure 1. An oncogenic driver was identified in 44% of samples. Overall survival was improved substantially with a driver with bioguided treatment (HR, 0.5; 95% CI, 0.3 to 0.9; P = .014) compared with a driver without bioguided treatment or no driver identified. Patients with an ECOG PS of ≥ 2 were less likely to receive the driver with bioguided treatment; the incidence of brain metastases was similar in the 3 treatment groups. Prof Barlesi noted that a recent study in metastatic lung ADC conducted in the United States identified oncogenic drivers in 64% of patients and also found that matching therapy to the driver improved survival [Kris MG et al. JAMA. 2014].
Genetic Profile in Local Study of 262 Patients
Reproduced with permission from F Barlesi, MD.
In France, from 2015, there will be routine testing for molecular targets and matching of the treatment to the target, stated Prof Barlesi, and from 2017 there will be wider implementation of NGS. The results of the Biomarkers France study, including its ancillary studies in patients with specific mutations, may lead to a second such study.
Potential Molecular Targets for Squamous Cell Cancer of the Lung
Few molecular targets have been identified for squamous cell cancer of the lung. The NFE2L2 mutation is very frequent in squamous cell cancer of the lung, but there is no drug available to target it, according to Roman K. Thomas, MD, University of Cologne, Cologne, Germany. Although DDR2 mutations appear to be difficult to target, it is worthwhile to screen for the S768R mutation because of 2 case reports of a response to dasatinib [Pitini V et al. Lung Cancer. 2013; Hammerman PS et al. Cancer Discov. 2011].
FGFR1 mutations are a potential target in small cell lung cancer, and FGFR1 amplification is seen in about 22% of cases [Weiss J et al. Sci Transl Med. 2010]. The finding that FGFR1 amplification was sensitive to inhibition with PD173074 [Malchers F et al. Cancer Discov. 2014] led to a phase 1 study conducted by Prof Thomas and colleagues through Network Genomic Medicine in Germany, which provided central testing and outreach to nonacademic hospitals and oncologists and pulmonologists in private practice. Survival data were obtained through the national census.
The study enrolled 4835 patients and successfully tested 3858 patients (79%). This is one of the largest single-center trials in the world in this patient population and includes about 80% of the lung cancer patients in the region and about 10% of those in Germany. The novel compound BGJ398, a highly selective FGFR1 inhibitor given as second- or third-line treatment, elicited a response in 16% of patients [Sequist LV et al. AACR 2014 (abstr CT326)]. This supports the notion that FGFR1 amplification is a treatable target in small cell lung cancer, stated Prof Thomas, and the responses were somewhat durable. The patient population who will benefit must be better discriminated.
Lung-MAP: Master Protocol for Clinical Trials in Lung Cancer
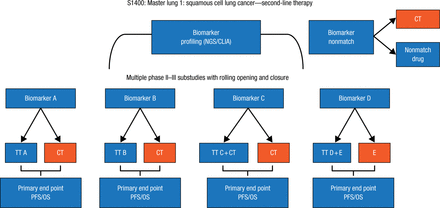
The Lung-MAP study [NCT02154490] represents a new model for clinical trials that will allow for the simultaneous testing of novel compounds and predictive biomarkers. Patients with squamous cell lung cancer will undergo NGS and then be matched to 1 of several phase 2 and 3 substudies of novel compounds against molecular targets (Figure 2), stated David R. Gandara, MD, University of California Davis Comprehensive Cancer Center, Davis, California, USA. Each substudy is designed for registration of new drug-biomarker combinations. The master protocol allows for 1 screening, registration, and testing process of patients; a rolling open and closing of each substudy, which is independent; a control group for each substudy; and the flexibility to amend the study as needed, such as when a new drug is approved.
Master Protocol for Lung-MAP Study of Squamous Cell Lung Cancer
CLIA, Clinical Laboratory Improvement Amendments; CT, chemotherapy (docetaxel or gemcitabine); E, erlotinib; NGS, next-generation sequencing; OS, overall survival; PFS, progression-free survival; TT, targeted therapy.
Reproduced with permission from DR Gandara, MD.
Lung-MAP, initiated in July 2014, is being conducted at about 500 sites in the National Clinical Trials Network, including Canada. It represents a unique public-private partnership between (1) the US National Cancer Institute, Southwest Oncology Group, Friends of Cancer Research, and Foundation for the National Institutes of Health, which handles the funding, and (2) pharmaceutical companies, which contribute about 20% of the usual cost of a phase 3 trial. This partnership resulted from a workshop led by the US National Cancer Institute to address the challenges of clinical trials in lung cancer because of its tumor heterogeneity and genomic complexity, which has led to mostly negative trials, the number of candidate molecular targets, and the slow pace for drug approvals with the classical approach to clinical trials. The master protocol approach also provides for developing drugs for genotypes that are rare or uncommon. Lung-MAP is anticipated to be self-sustaining, with new substudies being planned for new molecular targets and to adjust to the changing therapeutic landscape, stated Dr Gandara. Other studies using the master protocol approach are being conducted.
- © 2015 SAGE Publications