Summary
This randomized, phase 2 trial evaluated PGG beta-glucan, a novel immune modulator, in combination with bevacizumab and carboplatin/paclitaxel as first-line treatment for patients with stage IV non–small cell lung cancer. Though not statistically significant, the results for the primary and secondary end points favor the PGG beta-glucan–treated group and warrant further study of PGG beta-glucan as an adjunct to bevacizumab-containing chemotherapy regimens.
- non–small cell lung cancer
- stage IV
- PGG beta-glucan
- bevacizumab, immune cell modulator
- carboplatin/paclitaxel
- oncology clinical trials
Recent reports show that PGG beta-glucan, a novel immune cell modulator, increased objective response rates in patients with stage IV non–small cell lung cancer (NSCLC) when added to the first-line regimen of carboplatin/paclitaxel (C/P) chemotherapy and cetuximab, an EGFR-targeted antibody [Schneller F. J Thorac Oncol. 2014]. Bevacizumab is a vascular endothelial growth factor targeted antibody, approved in both the United States and Europe, for first-line treatment of unresectable, locally advanced, recurrent, or metastatic NSCLC when administered in combination with C/P chemotherapy.
Ada Braun, MD, PhD, Biothera, Eagan, Minnesota, USA, and colleagues presented results from a multicenter, open-label, randomized phase 2 trial [Braun A et al. Ann Oncol. 2015] investigating the efficacy of PGG beta-glucan when added to bevacizumab and C/P combination therapy for previously untreated stage IV nonsquamous NSCLC. The primary end point for this study was an objective response rate, whereas the secondary end points included duration of response, progression-free survival, time to progression, overall survival, and safety.
The investigators enrolled 92 patients between 2009 and 2013 from 12 centers across Germany and the United States, with histologically or cytologically confirmed, previously untreated, stage IV nonsquamous NSCLC. Eligibility required adequate organ function (hematologic, hepatic, renal, coagulation) and an ECOG performance status (PS) of 0 or 1. Patients were randomized 2:1 to bevacizumab plus PGG (n = 61; PGG 4 mg/kg intravenously; day 1, 8, 15 of each cycle; PGG beta-glucan group) or bevacizumab alone (n = 31; control group) in combination with C/P for 4 to 6 cycles until documented progression or unacceptable toxicity. All patients had either progressed or had completed at least 18 treatment cycles.
Baseline patient characteristics including age, sex, race, time from diagnosis to randomization, and prior treatments (surgery, radiation therapy) were balanced between the PGG beta-glucan and control treatment groups. A higher proportion of patients in the control group than in the PGG beta-glucan group had a good ECOG PS of 0 at baseline (66.7% vs 52.5%). Thus, a better clinical prognosis and clinical outcomes would have been expected in the patients in the control group, because ECOG PS 0 is a major prognostic factor, according to Prof Braun.
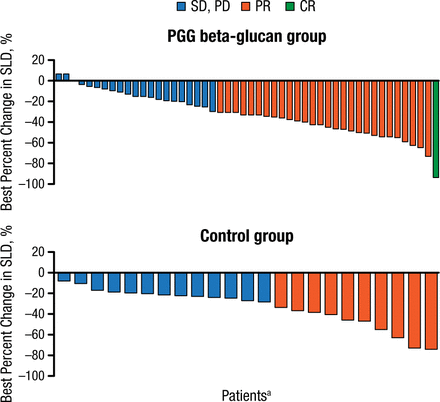
An objective response was achieved in 29 of 48 (60.4%; 95% CI, 45.3 to 74.2) patients in the PGG beta-glucan group and in 10 of 23 (43.5%; 95% CI, 23.2 to 65.5) patients in the control group (P = .2096; Figure 1). The investigators reported that the tumor size continued to regress post chemotherapy in the PGG beta-glucan maintenance group.
Best Overall Response (Change in Tumor Size) in PGG Beta-Glucan vs Control Group
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease;
SLD, sum of the longest diameters.
aEach bar shows the results for a single patient.
Reproduced with permission from A Braun, MD, PhD.
In the PGG beta-glucan and control groups, respectively, the median duration of response was 10.3 months and 5.6 months (P = .9040); the time to disease progression was 11.6 months and 9.6 months (HR, 1.31; 95% CI, 0.54 to 3.65; P = .5639); and progression-free survival was 11.9 months and 10.2 months (HR, 0.86; 95% CI, 0.49 to 1.54; P = .5901).
The overall survival was a median 11.6 months in the control group and 16.1 months in the PGG beta-glucan group (HR, 0.66; 95% CI, 0.38 to 1.16; P = .1345); the study was not powered to detect a statistical difference in survival between the study groups.
The incidence of adverse events (AEs) was similar between the treatment arms; however, 37.3% of the PGG beta-glucan cohort and 43.3% of the control cohort discontinued the study due to AEs. PGG beta-glucan-related AEs included chills (13.6%); dyspnea and fatigue (10.2% each); and nausea, pyrexia, and infusion-related reactions (8.5% each). The investigators concluded that PGG beta-glucan showed promise as an adjunct to antibody-based therapies for improving clinical outcomes in patients with NSCLC.
- © 2015 SAGE Publications