Summary
The phase 2b results of the randomized, double-blind, placebo-controlled TIME study provide evidence of activity with TG4010, a therapeutic cancer vaccine, when added to first-line chemotherapy for stage IV non–small cell lung cancer, especially for nonsquamous histology and low levels of triple-positive activated lymphocytes. These data suggest that normal levels of triple-positive activated lymphocytes may be a predictive biomarker of TG4010 efficacy.
- advanced non–small cell lung cancer
- immunotherapy
- TG4010
- cancer vaccine, triple-positive activated lymphocytes
- predictive biomarkers
- TIME study
- oncology clinical trials
Lung cancer is emerging as a promising target for immunotherapy, with the PD-1 inhibitor nivolumab just recently approved by the FDA. Two categories of novel immunotherapies being evaluated include cancer vaccines and checkpoint inhibitors [Guibert N et al. Ther Adv Respir Dis. 2015]. Elisabeth Quoix, MD, The University Hospitals of Strasbourg, Strasbourg, France, and colleagues shared phase 2b results from TIME, a phase 2b/3 randomized, double-blind, placebo-controlled study comparing the efficacy of adding TG4010, a therapeutic cancer vaccine, to first-line treatment for stage IV non–small cell lung cancer (NSCLC) [Quoix E et al. Ann Oncol. 2015]. TG4010 is a modified attenuated poxvirus (Ankara strain) coding for MUC1 tumor-associated antigen and interleukin-2. The aim of the phase 2b study was to validate a normal level of triple-positive activated lymphocytes (TrPALs; including CD16+, CD56+, and CD69+) as a predictive biomarker for TG4010 efficacy. The primary end point was progression-free survival (PFS) assessed by RECIST 1.1, while the secondary end points included overall response rates, safety, overall survival (OS), and subgroup analysis.
The trial enrolled 221 patients with untreated NSCLC and an MUC1 mutation. Patients were stratified by TrPAL levels (normal vs high) and then randomized 1:1 to receive TG4010 (subcutaneous injection, 108 PFU weekly over 6 weeks, once every 3 weeks thereafter until progression) or placebo in combination with chemotherapy (21-day cycles for 4-6 cycles). Bayesian analysis of PFS in patients with normal TrPAL levels (n = 170) treated with TG4010 (n = 85) or placebo (n = 85) was conducted after 144 events of disease progression.
In the patients with normal TrPAL levels, the primary end point of PFS was achieved in 70 patients (82.4%) receiving TG4010 and 74 patients (87.1%) receiving placebo. The observed hazard ratio (HR) for PFS was 0.74 (95% CI, 0.53 to 1.02), which corresponded to a 98.6% Bayesian probability that the true HR was < 1 and thus passed the necessary threshold of 95% to have met the efficacy end point in the normal TrPAL patient population. In patients with normal TrPAL levels, median PFS favored the TG4010 treatment arm compared with standard chemotherapy alone (5.7 vs 5.1 months, respectively; HR, 0.78; 95% CI, 0.53 to 1.02; P = .078). The overall response rate was 37.6% in the TG4010 treatment arm compared and 30.6% in the placebo arm of the patients with a normal TrPAL level. The analysis of patients with high TrPAL levels is still pending.
Most grade 3/4 adverse events were similar between the treatment arms and included neutropenia, thrombocytopenia, fatigue, anemia, and febrile neutropenia with higher TG4010-related adverse events at the injection site (31.4% vs 4%).
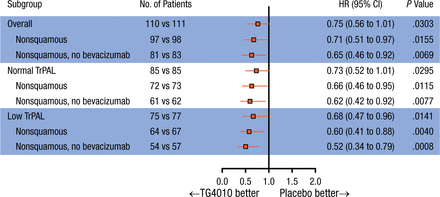
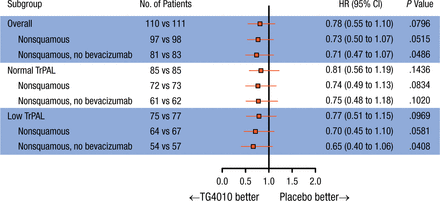
Subgroup analyses in patients with nonsquamous NSCLC (n = 195) showed a significant improvement in PFS when treated with TG4010 (HR, 0.71; 95% CI, 0.51 to 0.97; P = .016), with an increase in OS (HR, 0.73; 95% CI, 0.50 to 1.07). In the 75% of patients with the lowest baseline TrPAL levels (low TrPAL; n = 152), the HR for PFS was 0.66 (95% CI, 0.46 to 0.96; P = .014). In the patients with nonsquamous NSCLC and low TrPAL levels (n = 131), PFS was significantly increased in the TG4010 arm vs placebo (HR, 0.60; 95% CI, 0.41 to 0.88) and OS was increased with TG4010 vs placebo (HR, 0.70; 95% CI, 0.45 to 1.10). Forest plots of PFS and OS in the stratified subgroups are shown in Figures 1 and 2.
Progression-Free Survival by Subgroup
The HR is from the unstratified Cox proportional hazards model. The P value (one-sided) is from the unstratified log-rank test.
TrPAL, triple-positive activated lymphocyte.
Reproduced with permission from E Quoix, MD.
Overall Survival by Subgroup
The HR is from the unstratified Cox proportional hazards model. The P value (one-sided) is from the unstratified log-rank test.
TrPAL, triple-positive activated lymphocyte.
Reproduced with permission from E Quoix, MD.
Prof Quoix concluded that the results of the phase 2b portion of the TIME study provided evidence of the efficacy and safety of TG4010 in stage IV NSCLC, especially in patients with nonsquamous tumors and low TrPAL levels.
- © 2015 SAGE Publications