Summary
PEGASUS-TIMI 54 and DAPT trials demonstrated that dual antiplatelet therapy reduced the risk of cardiovascular death, myocardial infarction, or stroke among patients with a history of myocardial infarction, and reduced the risk of ischemic events in patients receiving coronary stents when compared with treatment with aspirin alone but is associated with significantly increased risk of bleeding complications.
- antiplatelet therapy
- DAPT
- myocardial infarction
- P2Y12 receptor antagonist
- PEGASUS-TIMI 54
- stent
- stroke
- ticagrelor
- TIMI
- thienopyridine
- NCT00526474
- NCT00977938
- cardiology & cardiovascular medicine clinical trials
The use of dual antiplatelet therapy (DAPT) in which a P2Y12 receptor antagonist is combined with aspirin is recommended to reduce the risk of ischemic events or thrombosis in some at-risk patient populations. However, whether prolonged therapy may be beneficial for some patients has not been fully elucidated. Marc S. Sabatine, MD, Brigham and Women’s Hospital, Boston, Massachusetts, USA, presented results of the PEGASUS-TIMI 54 trial [Bonaca MP et al. N Engl J Med. 2015], demonstrating that the addition of ticagrelor to low-dose aspirin reduced the risk of cardiovascular (CV) death, myocardial infarction (MI), or stroke, and increased the risk of TIMI major bleeding among patients with a history of MI. Robert W. Yeh, MD, Massachusetts General Hospital, Boston, Massachusetts, USA, presented results of a post hoc subgroup analysis [Yeh RW et al. J Am Coll Cardiol. 2015] of the DAPT Study showing that continuation of a thienopyridine plus aspirin vs aspirin alone beyond 1 year reduced the risk of ischemic events in patients with and without acute coronary syndromes (ACSs) receiving coronary stents.
PEGASUS-TIMI 54 was a multicenter, international, randomized, double-blind, placebo-controlled clinical trial that investigated whether long-term therapy with ticagrelor would reduce the risk of major adverse CV events in stable patients with a history of MI receiving low-dose aspirin. The primary efficacy end point was the composite of CV death, MI, or stroke. Secondary end points were CV death and all-cause death. The primary safety end point was TIMI major bleeding. Other safety end points included intracranial hemorrhage and fatal bleeding.
A total of 21,162 patients were randomized: 7050 received ticagrelor 90 mg BID, 7045 received ticagrelor 60 mg BID, and 7067 received placebo. The median follow-up was 33 months.
At 36 months, ticagrelor significantly reduced the rate of the primary composite end point at both doses, with 9.0% of patients in the placebo group experiencing an event vs 7.8% in both groups receiving ticagrelor (90 mg BID: HR, 0.85; 95% CI, 0.75 to 0.96; P = .008, and 60 mg BID: HR, 0.84; 95% CI, 0.74 to 0.95; P = .004). Dr Sabatine noted that the event curves for both ticagrelor groups separated early (within 2 to 3 months) from the placebo event curve and continued to diverge over time. Treatment with ticagrelor was also consistently favored when individual components of the primary end point were analyzed separately and in prespecified patient subgroups.
The rate of TIMI major bleeding was significantly higher with the 2 ticagrelor doses as compared with placebo; 2.6% in patients receiving ticagrelor 90 mg (HR, 2.69; 95% CI, 1.96 to 3.70; P < .001), 2.3% in those receiving ticagrelor 60 mg (HR, 2.32; 95% CI, 1.68 to 3.21; P < .001), and 1.1% in those on placebo. However, the rates of fatal bleeding or nonfatal intracranial hemorrhage did not differ significantly between either ticagrelor group and placebo (P = .47). Dyspnea occurred significantly more frequently with ticagrelor 90 mg and 60 mg than with placebo (18.9%, 15.8%, and 6.4%, respectively; P < .001). Gout also was significantly more frequent with ticagrelor 90 mg (2.3%; P < .001) and 60 mg (2.0%; P = .01) than with placebo (1.5%).
Dr Sabatine concluded that the addition of ticagrelor to low-dose aspirin in stable patients with a history of MI reduced the risk of CV death, MI, or stroke and that long-term DAPT with low-dose aspirin and ticagrelor should be considered in appropriate patients with MI.
The DAPT Study was a multicenter, international, prospective, randomized, double-blind, clinical trial that showed 30 months vs 12 months of DAPT in patients who had coronary stenting was more effective in reducing the primary end points of stent thrombosis and the composite of death, MI, or stroke, but it was associated with a significantly increased risk of moderate or severe bleeding [Mauri L et al. N Engl J Med. 2014].
In this post hoc subgroup analysis of the 11,648 patients randomized at 12 months in the DAPT Study, 3576 patients had an ACS (1805 continued thienopyridine, 1771 received placebo) and 8072 patients did not have an ACS (4057 continued thienopyridine, 4015 received placebo) [Yeh RW et al. J Am Coll Cardiol. 2015]. The patients with ACS were younger and had fewer comorbidities than the patients without ACS. In both groups, approximately one-third of patients randomized to a thienopyridine received prasugrel and two-thirds received clopidogrel.
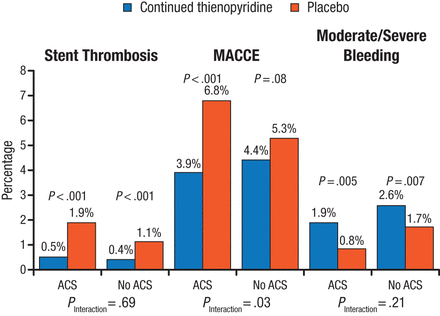
Continued thienopyridine therapy significantly and consistently reduced the rate of stent thrombosis in both patients with ACS (0.5% vs 1.9% on thienopyridine vs placebo, respectively; HR, 0.27; 95% CI, 0.13 to 0.57; P < .001) and without ACS (0.4% vs 1.1% on thienopyridine vs placebo, respectively; HR, 0.33; 95% CI, 0.18 to 0.60; P < .001; Figure 1). The rate of the composite of death, MI, and stroke was reduced significantly among patients with ACS (3.9%; P < .001 vs placebo), but no significant difference was found among patients without ACS (4.5%; P = .09 vs placebo). Moderate and severe bleeding was consistently increased by thienopyridine therapy among both patients with ACS (1.9% vs 0.8% with placebo; HR, 2.38; 95% CI, 1.27 to 4.43; P = .005) and patients without ACS (2.6% vs 1.7% with placebo; HR, 1.53; 95% CI, 1.12 to 2.08; P = .007).
Primary End Points of the DAPT Study at 12 to 30 Months
ACS, acute coronary syndrome; MACCE, major adverse cardiac and cerebrovascular event.
Reproduced with permission from RW Yeh, MD.
Source: Yeh RW et al. J Am Coll Cardiol. 2015.
Dr Yeh concluded that DAPT with a thienopyridine plus aspirin beyond 1 year reduced the risk of stent thrombosis for all patients and major adverse cardiac and cerebrovascular events among patients with ACS compared with treatment with aspirin alone, and continuation of DAPT for 30 months should be strongly considered in the appropriate patient.
- © 2015 SAGE Publications