Summary
PARTNER 1 demonstrated that patients at high risk of surgery had a similar mortality at 5 years with transcatheter versus surgical aortic valve replacement, while the CoreValve US Pivotal Trial showed that transcatheter aortic valve replacement reduced mortality at 2 years when compared with surgical aortic valve replacement. Observational data showed that patients treated with MitraClip had improvements in mitral regurgitation. The AATAC-AF study showed that catheter ablation is superior to amiodarone in treating persistent atrial fibrillation.
- AATAC-AF
- amiodarone
- catheter ablation
- CoreValve US Pivotal Trial
- PARTNER 1
- surgical aortic valve replacement
- Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy registry
- transcatheter aortic valve replacement
- transcatheter mitral valve repair
- NCT00530894
- NCT01240902
- NCT0072991
- cardiology & cardiovascular medicine clinical trials
- interventional techniques & devices
The results of trials of the percutaneous treatment of aortic stenosis (AS) and mitral regurgitation (MR), including PARTNER 1 and CoreValve US Pivotal, showed a reduction in mortality, and registry data supported the safety of the MitraClip System. The AATAC-AF study showed that catheter ablation was superior to amiodarone for the treatment of persistent atrial fibrillation (AF).
Percutaneous Treatment of Valvular Disease
Michael Mack, MD, The Heart Hospital Baylor Plano, Plano, Texas, USA, reported the results from long-term follow-up of the PARTNER 1 trial [Mack MJ et al. Lancet. 2015]. At 5 years, high-surgical-risk patients with severe AS who underwent transcatheter aortic valve replacement (TAVR) had similar mortality and other major clinical outcomes to those who were treated with surgical aortic valve replacement (SAVR).
The PARTNER 1 trial was an international, multicenter, randomized controlled trial that randomized patients who were at high surgical risk to either TAVR with the Edwards Sapien valve or SAVR. The primary end point of the trial was mortality at 1 year. Other key end points included valve performance and stroke rate.
A total of 699 patients were randomized to either TAVR (n = 348) or SAVR (n = 351). The median survival was 44.5 months in the TAVR group vs 40.6 months in the SAVR group. All-cause mortality at 5 years was not significantly different between the groups, with a rate of 68% in the TAVR group vs 62% in the SAVR group (HR, 1.04; 95% CI, 0.86 to 1.24; P = .76).
There was also no significant difference between the groups in the rate of CV mortality, stroke, or rehospitalization. NYHA class, valve hemodynamics, and improvements in valve function were maintained in both groups at 5 years.
Dr Mack concluded that the results with TAVR are similar to those seen with SAVR at 5 years. Thus, TAVR is a viable alternative to surgery in high-surgical-risk patients, as patients treated with TAVR had similar mortality and other major clinical outcomes, including stroke, to those treated with SAVR.
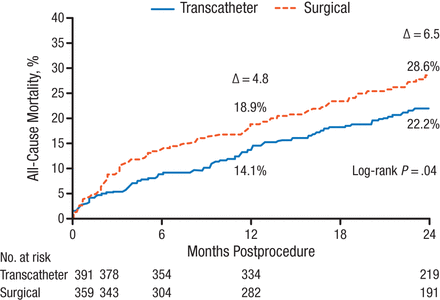
Michael J. Reardon, MD, Houston Methodist Hospital, Houston, Texas, USA, presented the 2-year follow-up results of the CoreValve US Pivotal Trial [NCT01240902] that demonstrated TAVR using the Medtronic CoreValve reduced mortality when compared with SAVR in patients with severe AS.
The CoreValve US Pivotal Trial [Adams DH et al. N Engl J Med. 2014] was a multicenter, randomized, noninferiority comparison study of the self-expanding transcatheter CoreValve with SAVR in patients with severe AS deemed at increased risk for surgery. The primary end point analysis, all-cause mortality at 1 year, was 14.2% in the TAVR group and 19.1% in the SAVR group (PSuperiority = .04).
The as-treated population for 2-year results included 750 patients: 391 underwent attempted TAVR, and 359 underwent attempted SAVR. The median follow-up was 24 months. Key end points included 2-year mortality, neurological events, major adverse cardiac and cerebrovascular events, and echocardiographic outcomes.
At 2 years, all-cause mortality was 22.2% in the TAVR group and 28.6% in the SAVR group (log-rank P = .04; Figure 1). TAVR was also favored in all prespecified subgroup analyses.
All-Cause Mortality at 2 Years in CoreValve US Pivotal Trial
Reproduced with permission from MJ Reardon, MD.
The rate of stroke at 2 years was 10.9% in the TAVR group and 16.6% in the SAVR group (log-rank P = .05), while the difference in the rate of major stroke was not significant (log-rank P = .25). The rate of major adverse cardiac and cerebrovascular events was 29.7% in the TAVR group and 38.6% in the SAVR group (log-rank P = .01). Echocardiographic findings revealed that patients who underwent TAVR had significantly better valve performance over those who underwent SAVR at all follow-up visits (P < .001).
Dr Reardon concluded that the improvements in survival seen at 1 year for patients treated with TAVR over SAVR were maintained at 2-year follow-up. He also noted that he believes TAVR with the self-expanding valve should be considered as the preferred treatment in patients with symptomatic severe AS who are at increased risk for surgery.
Paul Sorajja, MD, Minneapolis Heart Institute at Abbott Northwestern Hospital, Minneapolis, Minnesota, USA, reported data from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (TVT) registry, revealing the outcomes of transcatheter mitral valve repair (TMVR) with the MitraClip system when used for the treatment of patients with symptomatic MR.
The number of US patients affected by moderate or severe MR is expected to reach 5 million by 2030 [Enriquez-Sarano M, Sundt TM. Circulation. 2010]. Surgery is the standard of care; however, the risk of surgery is prohibitive for some patients. TMVR with MitraClip received FDA approval in 2013 and is indicated for symptomatic patients with grade ≥ 3 MR who are at prohibitive surgical risk.
All commercial cases with MitraClip enrolled in the TVT registry were identified and examined for in-hospital and 30-day outcomes for procedural success (postimplant MR grade ≤ 2 without CV surgery and in-hospital mortality), complications (cardiac perforation, major bleeding, stroke, myocardial infarction, mitral injury, or death), and device-related adverse events (AEs).
A total of 564 patients (median age 83 years; 56% men) were identified at 61 hospitals. Severe comorbidities were common; 57.2% of patients were classified as frail, and 94% had MR grade 3 or 4.
Procedural success was achieved in 91.8% of patients, with a complication rate of 7.8% and device-related AE rate of 2.7%. In-hospital mortality was 2.3%, and mortality at 30 days was 5.8%. Mean hospital stay was 3 days, with 81.9% of patients discharged directly to home. After implantation, 93% of patients had MR grade ≤ 2 and 63.7% had MR grade ≤ 1.
Dr Sorajja noted that the population in the TVT registry was older and had a higher prevalence of degenerative MR compared with other registries. He concluded that this first report of initial commercial experience with TMVR in the United States demonstrated that TMVR with MitraClip was safe and effective for patients with symptomatic MR and prohibitive surgical risk.
Treatment of Persistent AF
Luigi Di Biase, MD, PhD, Texas Cardiac Arrhythmia Institute at St David’s Medical Center, Austin, Texas, USA, and Montefiore Medical Center, Bronx, New York, USA, presented the results of the AATAC-AF [NCT00729911] study, demonstrating that catheter ablation is superior to amiodarone in treating persistent AF and reduces hospitalizations and mortality in patients with heart failure.
AATAC-AF was a phase 4, multicenter, randomized, parallel-group study with the primary end point of long-term procedural success defined as freedom from AF, atrial flutter, or atrial tachycardia > 30 seconds while off antiarrhythmic drugs.
Patients (n = 203) were randomized either to catheter ablation (n = 102) or to amiodarone (n = 101). The baseline characteristics were similar between the groups, and all patients had ≥ 6-month follow-up. After the mean follow-up of 26 months, 70% of patients in the ablation group and 34% in the amiodarone group were free from AF recurrence (log-rank P < .0001). In the amiodarone group, 10.4% of participants had to discontinue due to AEs.
Among the 102 patients in the ablation group, 80 patients underwent pulmonary vein isolation (PVI) plus posterior wall and nonpulmonary vein trigger ablation, while 22 patients underwent PVI ablation alone. The success rate was higher in patients undergoing PVI plus ablation (78.8%) compared with PVI alone (36.4%; P < .001).
Predictors for AF recurrence were identified as amiodarone therapy (HR, 2.5; 95% CI, 1.5 to 4.3; P < .001) and diabetes mellitus (HR, 1.1; 95% CI, 1.07 to 1.26; P = .01).
The rate of hospitalization was significantly lower in the ablation group than in the amiodarone group (31% vs 57%, respectively; P < .001). All-cause mortality was also lower in the ablation group than in the amiodarone group (8% versus 18%, respectively; P = .037).
Dr Di Biase concluded that treatment of persistent AF with catheter ablation in patients with heart failure increased freedom from AF while reducing hospitalization and mortality. He cautioned that the potential socioeconomic implications of these results require further investigation.
- © 2015 SAGE Publications