Summary
Results from IMPROVE-IT showed that adding ezetimibe to statin therapy with simvastatin significantly reduced the primary end point in moderate- to high-risk patients stabilized after acute coronary syndromes. A new analysis from IMPROVE-IT showed that the number of first and recurrent primary end point events was also reduced with combination therapy vs monotherapy over the median 6-year follow-up.
- ezetimibe
- simvastatin
- statins
- cardiovascular disease
- IMPROVE-IT trial
- acute coronary syndrome
- cardiology & cardiovascular medicine clinical trials
- coronary artery disease
- myocardial infarction
According to results of a secondary analysis of the IMPROVE-IT study presented by Sabina A. Murphy, MPH, Brigham and Women’s Hospital, Boston, Massachusetts, USA, adding ezetimibe to simvastatin therapy significantly improved clinical outcomes beyond a first event compared with simvastatin alone. This analysis also confirmed the importance of continuing intensive combination lipid-lowering therapy after a first cardiovascular (CV) event.
Ezetimibe is a nonstatin lipid-lowering therapy that reduces cholesterol absorption in the intestine. When added to a statin, achievement of low-density lipoprotein cholesterol (LDL-C) levels < 70 mg/dL or < 100 mg/dL was approximately 20% higher compared with a statin alone [Morrone D et al. Atherosclerosis. 2012]. IMPROVE-IT [NCT00202878] was a phase 3, multicenter, randomized, double-blind, active-control trial that evaluated whether ezetimibe added to simvastatin improved CV outcomes compared with simvastatin therapy alone.
IMPROVE-IT included 18 144 moderate- to high-risk patients stabilized after acute coronary syndromes (≤ 10 days) receiving standard medical and interventional therapy. Patients with a LDL-C level between 50 and 125 mg/dL (or 50 to 100 mg/dL if they had been taking prior lipid-lowering therapy) were randomized in a 1:1 ratio to once-daily doses of either ezetimibe/simvastatin (10/40 mg) or simvastatin monotherapy (40 mg) and followed for 2.5 years or until at least 5250 patients experienced a primary end point event.
The primary end point of the first occurrence of CV death, nonfatal myocardial infarction (MI), rehospitalization for unstable angina (UA), coronary revascularization (occurring ≥ 30 days after randomization), or stroke occurred in significantly more patients in the simvastatin monotherapy arm vs combination therapy arm (34.7% vs 32.7%; HR, 0.94; 95% CI, 0.89 to 0.99; P = .016). The number needed to treat was 50.
The occurrence of a first event for each of the 3 prespecified secondary end points was also significantly higher with simvastatin monotherapy vs combination therapy. All-cause death/MI/UA/coronary revascularization/stroke occurred in 40.3% vs 38.7%, respectively (P = .034). Coronary heart disease (CHD) death/MI/urgent coronary revascularization occurred in 18.9% vs 17.5% (P = .016). CV death/MI/UA/any revascularization/stroke occurred in 36.2% vs 34.5% (P = .035). Significance was driven by fewer MIs, strokes, and urgent revascularization events.
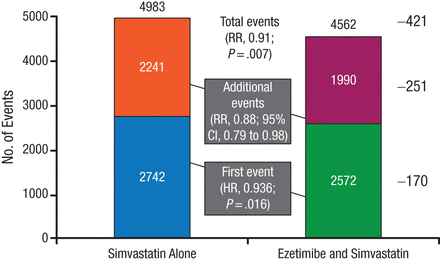
The present analysis determined the number of first and recurrent events recorded during the mean 6-year follow-up, with the hypothesis that the number of total events would be reduced with combination therapy vs simvastatin monotherapy. There were 5314 first primary end point events and 4231 additional primary end point events, the majority of which were revascularization for both first and recurrent events. Overall, there were significantly fewer total primary end point events with combination therapy (RR, 0.91; 95% CI, 0.85 to 0.97; P = .007; Figure 1). These results were reflected in a reduction in additional primary end point events (RR, 0.88; 95% CI, 0.79 to 0.98; Figure 1).
Fewer Total (First and Recurrent) Primary End Point Events With Combination Therapy
Reproduced with permission from SA Murphy, MPH.
There were fewer total secondary end point events with combination therapy as well, including fewer CHD deaths, MIs, and urgent revascularization events (RR, 0.85; 95% CI, 0.76 to 0.94; P = .002), fewer all-cause death/MI/UA/coronary revascularization/stroke (RR, 0.92; 95% CI, 0.87 to 0.98; P = .009), and fewer CV death/MI/UA/any revascularization/stroke (RR, 0.93; 95% CI, 0.87 to 0.99; P = .02).
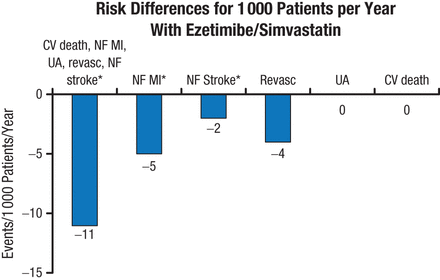
Sensitivity analysis using the Wei, Lin, and Weissfeld model for the occurrence of primary end point events favored combination therapy (model average HR, 0.93; 95% CI, 0.89 to 0.99; P = .01). The absolute risk difference for total primary end point events, nonfatal MI, and nonfatal stroke also favored ezetimibe/simvastatin therapy (P < .05; Figure 2).
Risk Differences for Total Primary End Point Events
CV, cardiovascular; MI, myocardial infarction; NF, nonfatal; Revasc, revascularization; UA, unstable angina.
*P < .05; others not significant.
Reproduced with permission from SA Murphy, MPH.
This is the first trial demonstrating clinical benefit when adding a nonstatin lipid-lowering agent to statin therapy. By treating patients with a daily combination of ezetimibe/simvastatin rather than simvastatin alone, more than twice the number of recurrent adverse CV events was prevented compared with first events.
- © 2015 SAGE Publications