Summary
Transcatheter aortic valve replacement was not superior to surgical aortic valve replacement in the randomized NOTION trial of low-risk patients with aortic valve stenosis. The transcatheter procedure did not significantly reduce the composite rate of death from any cause, stroke, or myocardial infarction at 1 year compared with surgery.
- aortic stenosis
- surgical aortic valve replacement
- transcatheter aortic valve replacement
- NCT01057173
- NOTION
- cardiology & cardiovascular medicine clinical trials
- myocardial infarction
In the first “all-comers” trial to randomize low-risk patients with aortic valve stenosis to transcatheter aortic valve replacement (TAVR) or surgical aortic valve replacement (SAVR), TAVR was safe and effective but not superior to SAVR on the primary outcome, the composite rate of death from any cause, stroke, or myocardial infarction (MI) at 1 year. Hans Gustav Hørsted Thyregod, MD, Copenhagen University Hospital, Copenhagen, Denmark, presented results from the prospective, randomized, multicenter, nonblinded NOTION trial [Thyregod HGH et al. J Am Coll Cardiol. 2015].
In previous studies of patients with extreme-risk aortic stenosis (AS) (Society of Thoracic Surgeons [STS] score > 15%) not considered as candidates for SAVR, the rate of the composite end point of death from any cause was 50.7% for standard therapy vs 30.7% for TAVR at 1 year [Leon MB et al. N Engl J Med. 2010]. The rate of all-cause mortality or major stroke at 1 year was 26% for TAVR-treated patients vs a prespecified objective performance goal of 43% [Popma JJ et al. J Am Coll Cardiol. 2014]. In high-risk patients (STS score 10% to 15%) randomly assigned to TAVR or SAVR, rates of death from any cause at 1 year were 24.2% for TAVR vs 26.8% for SAVR [Smith CR et al. N Engl J Med. 2011] and 14.2% for TAVR vs 19.1% for SAVR [Adams DH et al. N Engl J Med. 2014].
The objective of the NOTION trial was to compare TAVR with SAVR in an all-comers population of surgery-eligible patients aged ≥ 70 years. Investigators randomized 280 patients (mean age 79 years; 53% men) with low-risk severe aortic valve stenosis (mean STS score 3%) who were expected to live > 1 year to TAVR (n = 145) or SAVR (n = 135). The primary outcome was a composite of death from any cause, stroke, or MI at 1 year. Secondary outcomes included safety and efficacy and echocardiographic outcomes. Baseline characteristics and comorbidities were not significantly different in the 2 groups.
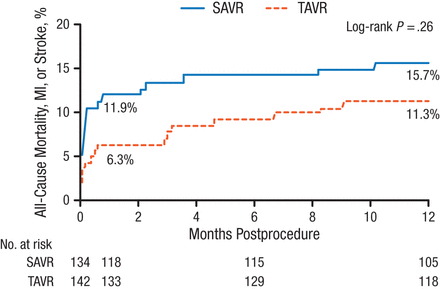
In intention-to-treat analysis, the composite rate of death from any cause, stroke, or MI at 1 year was 13.1% for TAVR vs 16.3% for SAVR (P = .43). In as-treated analysis, rates for the primary end point also failed to reach statistical significance (Figure 1).
Death From Any Cause, Stroke, or Myocardial Infarction at 1 Year in As-Treated Population
MI, myocardial infarction; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Reproduced with permission from HGH Thyregod, MD.
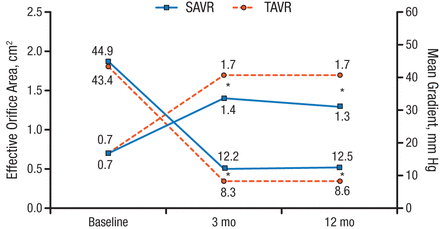
The only secondary outcomes to achieve statistical significance at 1 year were rates of atrial fibrillation (TAVR, 21.2% [n = 142]; SAVR, 59.4% [n = 134]; P < .001) and pacemaker implantation (TAVR, 38%; SAVR, 2.4%; P < .001). Among surviving patients at 1 year, 67.4% in the TAVR group (n = 132) were NYHA class I compared with 81.7% in the SAVR group (n = 120). A statistically significant difference (P < .001) favoring TAVR was seen for aortic valve performance (Figure 2). Rates of moderate-to-severe aortic valve regurgitation were 15.7% for TAVR vs 0.9% for SAVR at 1 year.
Aortic Valve Performance
SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
*P < .001.
Reproduced with permission from HGH Thyregod, MD.
In summary, the NOTION trial failed to demonstrate that TAVR was superior to SAVR for the primary outcome of the composite rate of death from any cause, stroke, or MI after 1 year in patients with low-risk severe AS. Dr Thyregod concluded that long-term durability and morbidity data were required in lower-risk patients.
- © 2015 SAGE Publications