Summary
As cerebrovascular vessel wall imaging techniques mature, new information will be provided useful for predicting the risk of stroke while increasing diagnostic specificity for a wide variety of common and uncommon intracranial circulatory disorders. Stroke risk prediction models are being revised to incorporate advances in optimized medical therapy and noninvasive imaging.
- magnetic resonance imaging
- carotid endarterectomy
- intracranial
- carotid artery
- imaging
- lumen
- vessel wall
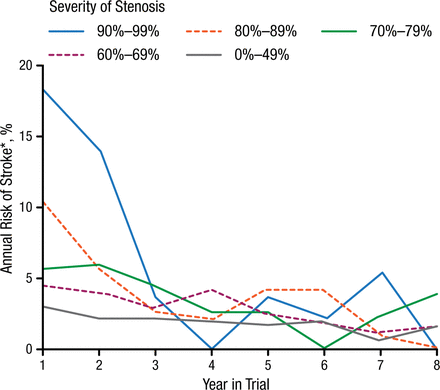
Several experts reviewed the role of modern cerebrovascular vessel wall imaging techniques in predicting the risk of stroke and diagnosing other disorders. Martin M. Brown, MD, University College London, London, United Kingdom, reviewed the randomized clinical trials of carotid endarterectomy (CEA) and provided a summary of risk prediction. Prof Brown stated that the data from several trials in the 1980s and 1990s remains relevant in studying predictors of future stroke. The final European Carotid Surgery Trial (ECST) results [ECST Collaborative Group. Lancet. 1998] showed that by 3 years there was no relationship between the severity of stenosis and annual risk of stroke (Figure 1). Risk modeling in ECST showed that clinical factors such as cerebral vs ocular events, plaque surface irregularity, and recent events were better predictors of recurrent events in patients on medical therapy than the degree of stenosis (Table 1) [Rothwell PM, Warlow CP. Lancet. 1999]. There is no evidence that stenosis severity predicts first stroke in patients with asymptomatic stenosis, said Prof Brown.
Annual Risk of Stroke With BMT Patients During Follow-up in ECST
BMT, best medical therapy; ECST, European Carotid Surgery Trial.
Note: Only strokes lasting > 7 days were recorded in ECST.
Reprinted from The Lancet, ECST, Randomized trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST), 1998;351:1372-1373. Copyright with permission from Elsevier.
*On May 1, 2015, Stoke was changed to Stroke.
Prognostic Variables From the European Carotid Surgery Trial
Since the time that ECST and other carotid surgery trials were conducted, medical therapy has improved, including advances in antiplatelet therapies, lower blood pressure control targets, and increased use of statins and cholesterol targets. According to Prof Brown, medical therapy alone has produced at least a 50% reduction of risk in asymptomatic stenosis causing symptoms over the next few years.
Risk scores are helpful in determining which patients are appropriate candidates for CEA or other interventions. The Carotid Artery Risk (CAR) score is the ECST prediction model that has been recalibrated to integrate the benefits of optimized medical therapy. This score predicts the 5-year risk of ipsilateral stroke in patients with > 50% carotid stenosis treated with optimized medical therapy alone [Kennedy F et al. Chirurg. 2013]. This method also includes asymptomatic stenosis and noninvasive stenosis imaging. The CAR score uses the following baseline characteristics: sex, degree of stenosis, plaque morphology (smooth or ulcerated), age, time since most recent event, most severe ipsilateral event (nondisabling stroke, retinal infarct, single or multiple transient ischemic attacks), diabetes, previous myocardial infarction, peripheral vascular disease, and hypertension. The CAR methodology still needs to be tested further and validated but will be available to clinicians in the future.
Thomas S. Hatsukami, MD, University of Washington, Seattle, Washington, USA, next discussed the clinical research supporting the use of carotid artery MRI in identifying patients at risk of stroke. Research has shown that angiography underestimates plaque burden, most likely due to expansive remodeling. Because of considerable heterogeneity in plaque composition, patients with similar degrees of stenosis can have very different plaque composition. Histologic studies have indicated that patients undergoing CEA that have an intact, thick fibrous cap (FC) tend to be asymptomatic, while a ruptured cap with intraplaque hemorrhage (IPH) is associated with symptoms prior to CEA [Mauirello A et al. Atherosclerosis. 2010]. MRI of carotid atherosclerosis has been validated by comparing preoperative in vivo images with histology of the specimens after CEA.
One study was undertaken to determine if carotid MRI could identify patients at increased risk for primary ischemic events [Takaya N et al. Stroke. 2006]. The 154 patients in this trial were initially asymptomatic, had 50% to 79% stenosis, underwent a carotid MRI at baseline, and had a clinical assessment every 6 months. Patients were followed for a mean of 38 months. Larger maximum wall thickness, IPH, and a thin or ruptured FC were associated with subsequent ipsilateral cerebrovascular events.
In a more recent study of 179 symptomatic patients with ≥ 50% carotid stenosis, carotid IPH at baseline MRI was a significant predictor of recurrent cerebrovascular events (P < .00001) [Hosseini AA et al. Ann Neurol. 2013]. Patients with 50% to 69% stenosis with baseline IPH had a cumulative annual risk of 40.6%, while the risk grew to 67.2% in those with 70% to 99% stenosis with IPH. The estimated absolute annual stroke risk in patients with no evidence of IPH in the carotid MRI was only 0.6%, said Dr Hatsukami. To date, evidence reported supports shifting from stenosis to identification of carotid plaques likely to hemorrhage in evaluating patient risk.
Lastly, David J. Mikulis, MD, University of Toronto, Toronto, Ontario, Canada, presented an overview of potential clinical and research applications for intracranial arterial wall imaging. He first highlighted case studies demonstrating the use of intracranial vessel wall imaging in a variety of intracranial circulatory disorders. From an applications perspective, Dr Mikulis next emphasized the need for high spatial resolution volumetric imaging, which he thought could be best achieved with a 3T MRI system.
Interpretation of wall imaging studies requires knowledge of how specific pathologic processes induce structural changes in the blood vessel wall, thus informing the diagnosis. There are characteristic pathologic features of the 3 main intracranial vascular diseases. Intracranial atherosclerotic plaque is characterized by eccentric wall thickening, increased T2 signal (particularly in the fibrous cap), variable gadolinium enhancement of the fibrous cap, and a lipid core that is hypointense to isointense compared with the brain. A T1 bright lipid core usually suggests IPH. High-risk plaque is characterized by vessel wall remodeling, irregularity of the plaque surface, IPH, high lipid volume, and contrast enhancement. Plaques that have positive remodeling and luminal narrowing are considered to have the highest risk for ischemic injury.
Secondly, dissection is characterized by eccentric luminal narrowing (less likely with subadventitial dissection) and T1 bright methemoglobin in the vessel wall. Lastly, vasculitis is characterized by luminal narrowing with diffuse, smooth, concentric wall thickening and enhancement.
A forthcoming white paper sponsored by the American Society of Neuroradiology will provide a comprehensive review of intracranial wall imaging with recommendations concerning the clinical application of vessel wall MRI. The objective of this initiative is to enable clinicians to differentiate medical conditions with similar angiographic features, to develop ways to detect and characterize arterial disease that does not result in luminal narrowing, and to provide better ways to monitor intracranial circulatory disease activity and response to treatment.
- © 2015 SAGE Publications