Summary
After an acute stroke, neurologic deficit can be limited and functional recovery can be improved with neurorestorative therapies, including stem cells and exosomes that enhance the spontaneous remodeling that occurs in the brain, spinal cord, and throughout the body. Neurorestoration complements traditional approaches to treating the brain lesion, such as with a tissue plasminogen activator and endovascular therapy.
- bone marrow stromal cells
- exosomes
- microRNA
- functional recovery
- ischemic stroke
- neurologic deficit
- neuroplasticity
- neurorestoration
- spontaneous remodeling
- tissue plasminogen activator
Therapies designed to enhance endogenous neurorestorative processes within intact tissue have a greater potential to reduce neurologic deficit and improve functional recovery after an acute stroke compared with traditional neuroprotective treatments that focus on treating the lesion and containing the degree of damage, according to Michael Chopp, PhD, Henry Ford Hospital, Detroit, and Oakland University, Rochester, Michigan, USA.
Neurologic improvement is seen over time in the majority of patients who have had a stroke, but the biological substrate and multiple endogenous processes that are responsible for this improvement have not been fully investigated nor capitalized on to improve outcomes after stroke or neural injury. Dr Chopp reviewed the intrinsic restorative processes that are stimulated after a stroke, as well as preclinical work in his laboratory to amplify these processes.
Parallel forms of spontaneous motor and neurological recovery are found in preclinical animal models of ischemic stroke and in human stroke. In both animals and humans, a “symphony of recovery” is automatically activated after an injury. These restorative processes, which include neurogenesis, angiogenesis, and neurite remodeling, work in concert to greatly enhance recovery, said Dr Chopp.
This spontaneous recovery occurs in many organs in the body by generating stem cells that replace the decaying system. After a stroke, there is a remarkable proliferation of stem cells that are generated ipsilateral to the lesion along the ventricles and migrate to the sites of the lesion, where they interact with newly formed vasculature angiogenic processes to facilitate rewiring. Rewiring of the axons and dendrites, and oligodendrogenesis within the neurorestorative microenvironment of the central nervous system, determines the degree of recovery after a stroke.
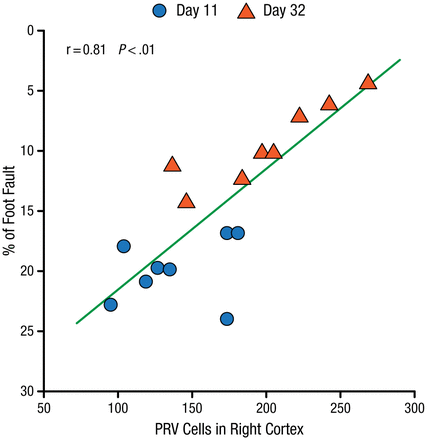
In an experimental study of mice with right middle cerebral artery occlusion (MCAo), there was a significant correlation between neuronal reorganization in the contralateral and ipsilateral hemispheres adjacent to the lesion. This was measured by the density of pyramidal neurons in the cortex after injection of pseudorabies virus into the stroke-impaired forelimb muscles, and the functional recovery was measured by the Foot-Fault test (P < .01; Figure 1) [Liu Z et al. Stroke. 2009].
Poststroke Functional Recovery Correlated With Cerebral Neuronal Reorganization
PRV, pseudorabies virus.
Reprinted from Liu Z et al. Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke. 2009;40:2546-2551. With permission from American Heart Association, Inc.
The ability of neurorestorative therapies to amplify the spontaneous rewiring after a stroke was shown by this group in a rat model of stroke injected with bone marrow stromal cells (BMSCs) [Liu Z et al. J Cereb Blood Flow Metab. 2010].The density of transcallosal axons in the contralateral cortex was significantly increased in rats subjected to MCAo compared with normal rats at day 28 after stroke, and this natural reorganization after a stroke was further increased with BMSC restorative treatment (P < .001). Additionally, neurological recovery after 28 days was correlated with contralateral cortical axonal sprouting and ipsilateral neuronal reorganization. Thus, the entire brain undergoes spontaneous remodeling, which contributes to improvement in neurologic deficits, stated Dr Chopp.
Spontaneous remodeling also occurs in the spinal cord. After the intravenous injection of BMSCs into rats with a right MCAo, there was a profound increase in neurite outgrowth from the right to the left cord, which also correlated with a significant improvement (P < .01) in neurologic function [Liu Z et al. Brain Res. 2007].
In addition to the neuroplasticity that occurs in the ipsilateral and contralateral hemispheres of the brain and in the spinal cord, there is a response to stroke throughout the entire body. For example, in a rat model of stroke, the expression of restorative genes in the bone marrow was significantly increased (P < .05) [Zacharek A et al. Stroke. 2010], showing that the entire body is responding to the brain lesion, stated Dr Chopp.
Significant improvement (P < .05) in neurologic function was obtained with a cell-based therapy that was injected 1 day [Chen J et al. Stroke. 2001], 1 week [Li Y et al. Glia. 2005], or 1 month [Shen LH et al. J Cereb Blood Flow Metab. 2007] after a malignant stroke was induced in a rat model. This work showed that the endogenous restorative processes can be amplified by a cell-based therapy that promotes neurovascular remodeling, well after the onset of stroke.
Restorative therapies, such as cell-based therapies for stroke, enhance neurovascular remodeling and promote neurological recovery. However, the mechanisms by which the restorative therapy accomplishes this remodeling have not been elucidated.
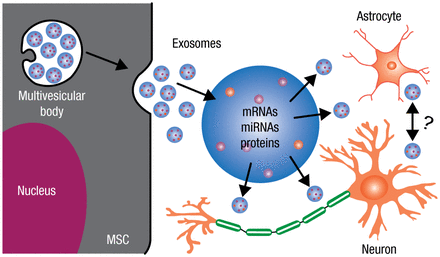
Chopp and his colleagues recently discovered that mesenchymal stromal cells (MSCs) mediate neurological recovery poststroke by emitting biological lipid nanoparticles (30 to 100 nm), referred to as exosomes. These exosomes contain proteins, messenger RNAs (mRNAs), and microRNAs. MicroRNAs, which are short, noncoding RNAs, act as “master molecular switches” and provide cellular instructions to simultaneously regulate hundreds of genes and their translation. Restorative therapies, such as cell-based therapy, work by releasing exosomes that contain protein and genetic instructions and information (microRNAs, mRNAs, and proteins), which then infect astrocytes, endothelial cells, and neurons, thereby dictating to the parenchymal cells what to do to restore neurologic function (Figure 2).
Exosomes Package and Transfer Information Between Cells
miRNA, microRNA; mRNA, messenger RNA; MSC, mesenchymal stromal cell.
Adapted from Li Y et al. The role of astrocytes in mediating exogenous cell-based restorative therapy for stroke. Glia. 2014;62:1-16. With permission from Wiley Periodicals, Inc.
Exosomes play a vital role in physiology and biology. Exosomes act as biological “exchange” particles that communicate information throughout the body. Thus, the presence of a lesion in the brain is communicated throughout the entire body, which activates the endogenous restorative processes.
Chopp and colleagues then showed that the direct administration of exosomes, derived from MSCs, into rats 24 hours after a stroke resulted in robust neurological recovery [Zhang Y et al. J Neurosurg. 2015; Xin H et al. J Cereb Blood Flow Metab. 2013]. Notably, the content of the exosomes can also be tailored by selecting specific microRNAs to turn on or off different processes, which is one of the ways that will lead to innovative restorative treatments.
In closing, greater functional recovery can be achieved with neurorestorative therapies that enhance the natural spontaneous plasticity that occurs after a stroke, neural injury, or neurodegenerative disease.
- © 2015 SAGE Publications