Summary
Cognitive dysfunction occurs after an aneurysmal subarachnoid hemorrhage, affecting psychosocial behavior, activities of daily living, and return to work. The mechanisms for the cognitive deficits are not understood, but neuroinflammation appears to be one process. New therapies to target neuroinflammation are being studied.
- aneurysmal subarachnoid hemorrhage
- cognitive dysfunction
- cognitive deficit
- heparin
- glibenclamide
- Mini–Mental Status Examination
- Montreal Cognitive Assessment
- neuroinflammation
- neuropsychological tests
- psychosocial behavior
- vasospasm
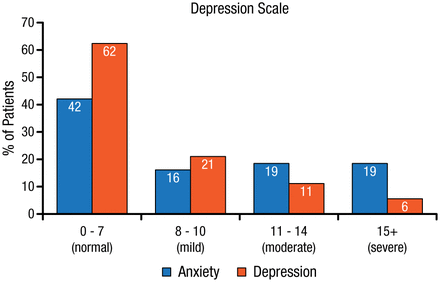
Cognitive function is affected after an aneurysmal subarachnoid hemorrhage (aSAH). A review of 61 studies identified deficits in memory, executive function, and language after an aSAH, which in turn affected activities of daily living, return to work, and quality of life [Al-Khindi T et al. Stroke. 2010]. Anxiety and depression were also increased in this patient population (Figure 1) [Morris PG et al. Neurosurgery. 2004]. However, there was a large variation in the proportion of patients who were affected across these studies, stated R. Loch Macdonald, MD, PhD, St Michael’s Hospital, Toronto, Ontario, Canada, which may be a result of the various neuropsychological tests (NPTs) used to assess cognitive functions.
Rates of Anxiety and Depression After Aneurysmal Subarachnoid Hemorrhage
Reprinted from Morris PG et al. Anxiety and depression after spontaneous subarachnoid hemorrhage. Neurosurgery. 2004;54:47-54. With permission from Congress of Neurological Surgeons.
Neuropsychological testing is beneficial to help the patient identify and understand the inability to return to work. Additionally, NPTs can be used to target rehabilitation. Within clinical trials, NPTs can be used for outcome assessment and possibly to understand the pathophysiology and mechanisms of the brain damage after an aSAH. However, there is a need for tests that are more sensitive and specific in assessing cognitive function and accurately detecting a treatment effect, compared with the modified Rankin Scale and Glasgow Outcome Scale, for example, which are cruder, broader measures.
The Mini–Mental Status Examination (MMSE) is short in duration but does not evaluate executive function after an aSAH. The Montreal Cognitive Assessment (MoCA) was developed to detect mild cognitive impairment, and it includes executive function and visual/spatial testing. MoCA was found to be more sensitive than MMSE to detect cognitive impairment and predict abnormalities in executive function, among others, in 2 studies [Wong GK et al. Eur J Neurol. 2014; Schweizer TA et al. J Neurol Sci. 2012] but was similar to MMSE in another study [Wong GK et al. J Neurol Neurosurg Psychiatry. 2012].
The development of additional NPTs may come from the common data elements project initiated by Prof Macdonald and colleagues, to reach consensus on a common set of cognitive tests that are short but comprehensive and validated in multiple languages and countries for multicenter data collection. They also started the Subarachnoid Hemorrhage International Trialists data repository to advance research in understanding cognitive dysfunction after an aSAH.
Mechanisms for Cognitive Dysfunction After an aSAH
The inflammatory system appears to play a role in the cognitive dysfunction and delayed onset of memory after an aSAH, but the mechanisms are not fully understood. Although vasospasm can lead to permanent deficit, other mechanisms are involved too, stated Jose Javier Provencio, MD, Cleveland Clinic Lerner Research Institute, Cleveland, Ohio, USA.
Subarachnoid hemorrhage (SAH) inhibits late long-term potentiation, known to affect spatial memory, through changes in the NMDA receptor that occur between days 3 and 6 after the hemorrhagic event. Neutrophils have also been implicated in the delayed deterioration post-SAH. One study showed an 8-fold increased risk of vasospasm in patients with cerebral spinal fluid (CSF) containing > 62% neutrophils on day 3 post-SAH [Provencio JJ et al. Neurocrit Care. 2010]. Neutrophils may cause delayed deterioration through their effects on blood vessels, red cell hemolysis, and reactive oxygen species production and direct effects on the brain and recruitment of more inflammatory cells.
The depletion of myeloid cells with anti-Lys6G/C antibody ameliorated delayed deterioration and vasospasm and improved behavioral testing in a mouse model [Provencio JJ et al. J Neuroimmunol. 2011]. More recently, Dr Provencio and colleagues found that the depletion of neutrophils at day 3 post-SAH in a mouse model led to improvement in NMDA receptor function and memory testing.
Neutrophils and monocytes leave the vasculature when an aneurysm ruptures, moving into the CSF in humans and mice. The neutrophils act on the microglial cells, neurons, and NMDA receptor. However, how the neutrophils cross over into the CSF is unknown, and this is the focus of an upcoming study in Dr Provencio’s laboratory. The 3-day delay in this process suggests a possible recruitment of systemic neutrophils as a second wave that is ultimately causing damage.
Strategies to Modulate Neuroinflammation Post-SAH
The activation of toll-like receptor 4 activates the microglia and release of tumor necrosis factor alpha (TNF-α), which has been linked to cognitive dysfunction in animal models. Compounds that block the sulfonylurea receptor 1–transient receptor potential melastatin 4 (Sur1-Trpm4) channel have been shown to reduce not only the levels of TNF-α and other markers of inflammation but also cognitive dysfunction in animal models of SAH, according to work reviewed by J. Marc Simard, MD, PhD, University of Maryland School of Medicine, Baltimore, Maryland, USA, and conducted in his laboratory. The Sur1-Trpm4 channel is upregulated in microglia, vessels, and astrocytes in animal models, and it was upregulated in neurons and vessels in human autopsy tissue.
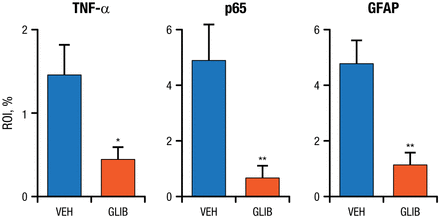
Glibenclamide has been shown to inhibit release of TNF-α from microglia activated by methemoglobin, and in another model of SAH it reduced TNF-α, transcription factor p65, and glial fibrillary acidic protein (Figure 2) [Simard JM et al. J Cereb Blood Flow. 2009]. Glibenclamide also has been shown to reduce induced nitric oxide synthase in microglia, as well as neutrophils and macrophages, and increase interleukin 10 in the entorhinal model of SAH. Notably, cognitive dysfunction was also reduced in the entorhinal model of SAH, with improvement in memory, rapid learning, and preservation of white and gray matter. These improvements are related to the beneficial effect of the anti-inflammatory agent in the hippocampal pathology, with better preservation of myelin and less cell death, stated Dr Simard.
Glibenclamide Reduced Markers of Inflammation in a Subarachnoid Hemorrhage Model
GFAP, glial fibrillary acidic protein; GLIB, glibenclamide; p65, transcription factor p65; ROI, region of interest; TNF-, tumor necrosis factor alpha; VEH, vehicle.
*P < .05.
**P < .01.
Adapted by permission from Macmillan Publishers Ltd: J Cereb Blood Flow. Simard JM et al. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. 2009;29:317-330. Copyright 2009.
Heparin is also a potent anti-inflammatory agent. Beneficial effects on cognitive function with heparin were found in a literature review [Simard JM et al. Neurocrit Care. 2010], in preclinical data, and in patients, according to Robert F. James, MD, University of Louisville, Louisville, Kentucky, USA.
In a rat model of SAH, heparin inhibited cortical inflammation and transsynaptic apoptosis and reduced demyelination [Simard JM et al. Transl Stroke Res. 2012]. In patients with an aSAH, a retrospective blinded review of 2 series of prospectively enrolled patients found that the incidence of clinical vasospasm (9% vs 47%; P = .0002) and infarcts on computed tomography (0% vs 21%; P = .003) was significantly lower in the heparin group compared with the control group, while the incidence of angiographic vasospasm was similar (58% vs 60%) [Simard JM et al. J Neurosurg. 2013]. The use of rescue therapy for vasospasm was also significantly lower in the heparin group vs the control group (P = .016 for phenylephrine and P = .021 for microcatheter-directed therapy). More patients in the heparin group were discharged to home rather than to a rehabilitation center.
These overall findings have led to a multicenter randomized controlled trial that will evaluate the safety of heparin and its effect on the primary outcome of the mean MoCA score at 90 days and secondary outcomes. The phase 2 open-label blinded adjudication Aneurysmal Subarachnoid Hemorrhage Trial Randomizing Heparin study will begin enrollment in spring 2015.
- © 2015 SAGE Publications