Summary
The Multicenter, Randomized, Double-blind, Parallel Group, Active-controlled Study to Evaluate the Efficacy and Safety of LCZ696 Compared to Enalapril on Morbidity and Mortality of Patients With Chronic Heart Failure and Reduced Ejection Fraction [PARADIGM-HF; NCT01035255] is the largest trial ever conducted with patients with heart failure with a reduced left ventricular ejection fraction and was designed to replace the current standard of care. This article discusses the rationale and design of the PARADIGM-HF trial.
- Cardiology Clinical Trials
- Heart Failure
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
- Heart Failure
The Multicenter, Randomized, Double-blind, Parallel Group, Active-controlled Study to Evaluate the Efficacy and Safety of LCZ696 Compared to Enalapril on Morbidity and Mortality of Patients With Chronic Heart Failure and Reduced Ejection Fraction [PARADIGM-HF; NCT01035255] is the largest trial ever conducted with patients with heart failure (HF) with a reduced left ventricular ejection fraction (LVEF) and was designed to replace the current standard of care. Milton Packer MD, University of Texas Southwestern Medical Center, Dallas, Texas, USA, presented the rationale and design of the PARADIGM-HF trial.
The use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs), β-blockers, and aldosterone antagonists is associated with significant reductions in cardiovascular morbidity and mortality in patients with HF with reduced EF. Neprilysin is an enzyme that catalyzes the degradation of many vasodilator peptides, such as natriuretic peptides, bradykinin, adrenomedullin, enkephalins, substance P, calcitonin gene-related peptide, and vasoactive intestinal polypeptide. These peptides are hormones that play a role in fluid homeostasis and are released in the setting of volume or pressure overload. Therefore, inhibition of neprilysin may augment the effect of these peptides. Whether the addition of a neprilysin inhibitor to standard therapy could offer additional clinical benefit remains unclear.
Omapatrilat is a dual inhibitor of ACE and neprilysin. The Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events trial [OVERTURE] demonstrated no significant difference in death or hospitalization for congestive HF (CHF) in patients who required intravenous therapy as compared with enalapril monotherapy but did increase the risk of serious angioedema [Solomon SD et al. Am Heart J 2005]. However, a sub-analysis of the OVERTURE trial suggested that treatment with omapatrilat resulted in significantly fewer deaths or CHF hospitalization overall (HR, 0.89; 95% CI, 0.82 to 0.98; p=0.012) and cardiovascular death or hospitalization (HR, 0.91; 95% CI, 0.84 to 0.99; p=0.024). In addition, omapatrilat was administered once daily in this trial but was designed to be given twice daily (BID).
To address the shortcomings of omapatrilat, LCZ696 was developed. LCZ696 is a combination of the ARB valsartan and AHU 377, a neprilysin inhibitor without the anti-aminopeptidase activity believed to cause angioedema. In 2009, a Phase 3 trial evaluating LCZ696 (LCZ) in patients with heart failure was initiated [NCT01035255], without having conducted a Phase 2 trial.
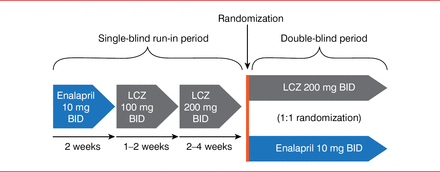
For the PARADIGM-HF trial, 8442 patients were enrolled. These patients had New York Heart Association (NYHA) Class II to IV heart failure with a LVEF of ≤40%, brain natriuretic peptide levels higher than or equal to 100 if hospitalized or less than or equal to 150 if not hospitalized within the past 12 months, a systolic blood pressure greater than or equal to 95 mm Hg, a glomerular filtration rate greater than or equal to 30 ml/min/1.73 m2, and serum potassium levels less than or equal to 5.4 mEq/L. Patients underwent a single-blind run-in period in which they received enalapril 10 mg BID for 2 weeks, then LCZ 100 mg BID for 1 to 2 weeks, and then LCZ 200 mg BID for 2 to 4 weeks (Figure 1). Patients then entered the double-blind period and were then randomly assigned to receive LCZ 200 mg BID or enalapril 10 mg BID. The primary end point was cardiovascular death or hospitalization for HF up to 4 years from the start of the trial.
Study Design
LCZ=LCZ696.
Adapted from McMurray JJ et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2013;15(9):1062–1073.
At baseline, the mean patient age was 64 years, 22% were women, and 66% were white. In this trial, 70% of patients had NYHA Class II HF, and the cause of LV dysfunction was ischemic heart disease in 60% of the participants.
In conclusion, the PARADIGM-HF trial was designed to establish a new standard of care for patients with CHF with a reduced LVEF. Dr. Packer concluded his presentation by highlighting that the PARADIGM-HF trial was stopped early due to the significant decrease in cardiovascular mortality.
- © 2014 MD Conference Express®