Summary
Inflammatory bowel disease is causally related to nutrition, particularly the westernized diet that emphasizes refined processed foods. But nutrition can be medicinal in pediatric and adult patients. Short-term replacement of food with a formulation or a modified diet can ease symptoms. A tailored diet can provide long-term relief, but this approach requires further rigorous study.
- diet
- IBD
- nutrition
- pediatric patients
Inflammatory bowel disease (IBD) is a chronic inflammation of any portion of the gastrointestinal tract. Two types of IBD, Crohn disease (CD) and ulcerative colitis (UC), are characterized by lifelong remissions and relapses. Malnutrition can manifest as weight loss, growth failure, and anemia. A session on nutritional therapy for IBD focused on the latest data and strategies to use nutrition to treat clinical symptoms and inflammation in patients with IBD.
Dale Lee, MD, Seattle Children’s Hospital, Seattle, Washington, USA, first discussed nutrition-based anti-inflammation therapy. IBD is more prevalent in industrialized nations that feature diets elevated in saturated fat, polyunsaturated fatty acids, and meat. The fact that second-generation immigrants have a higher risk of developing IBD than the prior generation has implicated the diet as a potential risk factor for the development of IBD [Li X et al. Inflamm Bowel Dis. 2010].
Tumor necrosis factor-α (TNF-α) has a central role in IBD [Neurath MF. Nat Rev Immunol. 2014]. Consequences of TNF-α on a variety of cell types include tissue destruction, angiogenesis, and hypervascularization. Anti-TNF-α therapy can promote mucosal healing in some, but not all, patients with IBD. Other strategies include corticosteroid- and immunomodulator-mediated immunosuppression and exclusive enteral nutrition (EEN).
In EEN, a formula-based diet provides the majority of the daily energy requirements, replacing solid-food consumption. The practice began in the 1930s and was substantiated by Voitk and colleagues in the 1970s. Studies in the decades since have found that EEN appears most effective for ileal or ileocolonic CD in the majority of children and some adults, but success is often hindered by poor compliance.
EEN formulations can reduce the level of inflammatory cytokines and promote mucosal healing, Dr Lee said; this may reflect avoidance of food and/or changes in the intestinal microbiome, since remissions tend to occur as formula is decreased and food is reintroduced. Research is focusing on the potential of certain foods to trigger intestinal inflammation; as yet, no specific recommendations have been determined.
Dr Lee went on to discuss the use of nutrition to manage IBD in pediatric patients, which is important given that one-quarter of all IBD cases are diagnosed by age 20 years, with a lifelong decline in incidence of IBD thereafter.
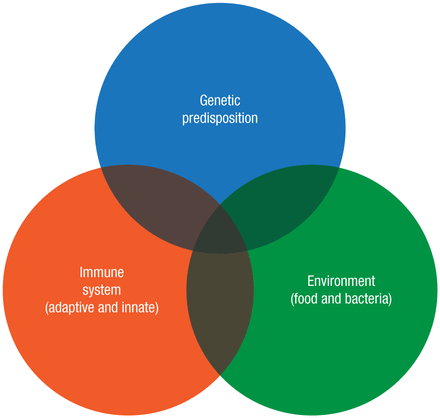
IBD involves the immune system, the environment, and the individual’s genetic proclivity (Figure 1). Conventional therapies work to suppress the immune system, whereas nutritional management works on environmental factors.
Etiology of IBD
IBD, inflammatory bowel disease.
Reproduced with permission from A.S.P.E.N.
Induction of remission involves corticosteroids and anti-TNF-α, with azathioprine and methotrexate used to maintain remission. This immune system–oriented approach does not address environmental factors and cannot address the underlying genetic contributors. Immunosuppression also increases the risks of infection, cancer, and drug-related side effects [Siegel CA. Gastroenterol Hepatol (NY). 2009].
EEN modifies the nutritional environment. A small randomized controlled trial comparing EEN and corticosteroids documented greater benefits in mucosal healing in the EEN group of pediatric patients with CD [Borrelli O et al. Clin Gastroenterol Hepatol. 2006]. A randomized controlled comparison of EEN with partial enteral nutrition (PEN), in which about half of the daily calories were derived from solid food, revealed the superiority of EEN in children with active CD [Johnson T et al. Gut. 2006]. The as-yet-unpublished, prospective-cohort PLEASE study compared outcomes of 8-week regimens of PEN, EEN, and anti-TNF-α therapies in children with IBD.
As compelling as these improvements are, complete solid-food exclusion is a short-term therapy. In the longer term, PEN has the potential to maintain clinical remission of CD compared with untreated patients [Yamamoto T et al. Inflamm Bowel Dis. 2007], and it has been found to be as effective as and less toxic than 6-mercaptopurine-mediated immunosuppression [Hanai H. Dig Liver Dis. 2012]. Another factor to consider is changes in the microbiome of the intestinal tract with EEN, which may be a cause or an effect of inflammation [Leach ST. Aliment Pharmacol Ther. 2008].
The focus shifted to the potential of other alternative diets in IBD, as discussed by Jennifer Burgis, MD, Stanford University, Palo Alto, California, USA. The typical diet in North America, which emphasizes refined processed foods, has been implicated as a risk factor for IBD. Increased risk for CD and UC has been linked to consumption of diets high in total fat, omega-6 fatty acids, and animal proteins. But nutrition can also be medicinal; the risk for CD can be decreased by consuming more fruits and fiber, and for UC by eating more vegetables [Hou JK et al. Am J Gastroenterol. 2011].
The benefits of nutrition in IBD can be gauged only experimentally, and diet therapies and studies are challenging for many reasons, including compliance issues, difficulty with capturing and measuring data, and inability to blind patients or conduct placebo-controlled trials.
A diet enriched in specific carbohydrates and restricted in others was formulated in the 1950s to treat celiac disease. More recently, several small studies involving children with CD who adhered to this diet found evidence for improvements in parameters, including height, weight, body mass index, and various protein markers [Cohen SA et al. J Pediatr Gastroenterol Nutr. 2014; Suskind DL et al. J Pediatr Gastroenterol Nutr. 2014].
This traditional specific-carbohydrate diet has been modified with individual dietary tailoring, such as the exclusion of lactose and refined/processed carbohydrates and fortification with omega-3 fatty acids, oats, flax seeds, and cocoa. In one review of 11 patients, compliance with the diet was good, with all participants experiencing improved symptoms of IBD and reduced use of medications [Olendzki BC et al. Nutr J. 2014]. The benefits, if any, of the Gut and Psychology Syndrome diet and the Paleo diet on IBD are unclear. The influence of a variety of other diets and the effect of systematic food exclusion on IBD have been explored with mixed results, said Dr Burgis.
The science of nutrition-related treatment of IBD is being clarified. For patients, this information becomes part of an effort to produce success. The effort also includes patient awareness of the diet and its purpose, a solid support team that provides help and encourages achievements, and determination of a backup plan ahead of time.
- © 2015 SAGE Publications