Summary
Micronutrients including vitamins, trace elements, and other compounds are important for brain development and the proper functioning of organs. Depending on the organ, age, and micronutrient, too little or too much of a micronutrient can be deleterious. The consequences may persist and can hinder physical and mental development.
- iron
- deficiency
- growth and development
- micronutrients
- monitoring
- nutrition
- parenteral nutrition
- neurodevelopment
A deficiency or excess of micronutrients, including vitamins, trace elements, and other compounds can be deleterious to organ development and function prior to birth and throughout life. The effects can be temporary or long-lasting and may not resolve with resolution of the deficiency.
Michael K. Georgieff, MD, University of Minnesota, Minneapolis, Minnesota, USA, discussed the significance of iron in early neurodevelopment. Iron is required for the proper development and function of every cell and organ, including the brain. Cognitive and motor problems related to a lack of available iron can be temporary or long-lasting, even after sufficient iron levels are restored. The timing of iron deficiency during brain development is crucial concerning the type and degree of brain dysfunction [Lozoff B et al. Nutr Rev. 2006]. Because the brain is not homogeneous in structure and development, the effects of nutrient deprivation can vary, depending on when the deprivation occurs and how long it lasts. For example, sensory perception is hard-wired by birth, but cognitive functions continue to be established for over a decade after birth.
Iron is required for the proper function of various enzymes and hemoproteins in myelin production, neuronal/glial energy status, and neurotransmitter and receptor manufacture. According to Dr Georgieff, the influence of iron deficiency is especially profound during fetal development and soon after birth (when 60% of the body’s oxygen demand is due to the brain), from 6 months to 2.5 years of age, and in female adolescents. Neonates, infants, and children up to 2.5 years can experience long-term deficits despite iron repletion.
Iron deficiency in the mother or fetus has been linked with increased risks of schizophrenia [Insel BJ et al. Arch Gen Psychiatry. 2008], autism [Schmidt RJ et al. Am J Epidemiol. 2014], delayed cognition [Riggins T et al. Dev Neuropsychol. 2009], and poorer reflexes. These may indicate persistent hippocampus dependent circuitry and myelin impairments.
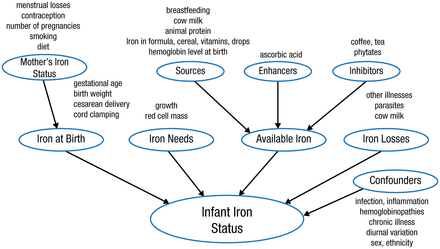
In infants and children (aged 6-24 months), iron status is governed by factors including the iron reserve at birth, requirement for iron, available iron, lost iron, and other factors (Figure 1). Iron deficiency in infants and children can lead to behavioral abnormalities and cognitive delays [Lozoff B et al. J Pediatr. 2008; Lozoff B et al. Nutr Rev. 2006] and electrophysiologic abnormalities associated with defective myelination [Algarín C et al. Pediatr Res. 2003].
Factors Determining Infant Iron Status
Adapted with permission of American Society for Nutrition, from Lozoff B et al. Iron deficiency in infancy: applying a physiologic framework for prediction. Am J Clin Nutr. 2006;84:1412-1421.
Human fetuses contain levels of iron that are higher than the typical levels present after the first several years of life. In the fetus, iron is sequestered mostly in red blood cells. When the mother is iron deficient or when iron availability is limited by decreased placental iron transfer or pregnancy-associated diabetes mellitus, the available iron is increasingly shunted to the red blood cells at the expense of the brain and other organs.
Edward Saltzman, MD, Tufts University, Boston, Massachusetts, USA, discussed how patients who undergo bariatric surgery can also experience micronutrient deficiency. According to Yanoff and colleagues, obesity is associated with abnormal iron deficiency in the blood [Int J Obes (Lond). 2007]. Sleeve gastrectomy is a weight loss measure for obese patients, in which a substantial portion of the stomach is removed. Factors that influence the risk for this micronutrient deficiency after sleeve gastrectomy include preoperative deficiency, inadequate nutrient intake following surgery, changes in digestion and absorption that result from reduced stomach volume, and inadequate nutrient intake following surgery. Bioavailability of iron is also diminished in obese patients due to the decreased tissue absorption of iron. This occurs because of the increased production of the liver peptide hormone hepcidin, which regulates iron absorption [Ganz T, Nemeth E. Annu Rev Med. 2011]. The persistent vomiting and inadequate food intake that can occur can provoke thiamine deficiency [Aasheim ET. Ann Surg. 2008].
A study of morbidly obese women who received a sleeve gastrectomy or Roux-en-Y gastric bypass documented marked reductions in heme and nonheme iron after both surgeries, with greater reductions in sleeve gastrectomy recipients [Ruz M et al. Am J Clin Nutr. 2012]. While many nutritional consequences can resolve with dietary supplementation, some will persist [Moizé Vet al. J Acad Nutr Diet. 2013]. Bariatric surgery can also disrupt bone homeostasis, which can lead to increased resorption [Folli F et al. Int J Obes (Lond). 2012]. Following bariatric surgeries, the intake and absorption of a variety of micronutrients can be diminished. The monitoring of micronutrient concentrations following surgery and adherence to dietary supplementation are both prudent to maintain physiologic levels of various micronutrients.
Another population at risk of altered micronutrient intake and absorption is the gut failure group who requires parenteral nutrition (PN) to meet all or most of their nutrient requirements. Gut failure is characterized by malabsorption resulting from inadequate bowel length or functional disability of available bowel. Carol J. Rollins, MS, RD, PharmD, The University of Arizona, Tucson, Arizona, USA, discussed the monitoring of micronutrients in patients receiving long-term PN.
Requirements for micronutrients depend on age, the disease, and the surgical history. Both the lack of micronutrients and toxic excess can be problematic. Problems may manifest as changes in physical appearance of skin, hair, and nails and altered gait. Biochemical changes can also occur. Deficiencies in essential fatty acids present signs and symptoms including alopecia, heightened fragility of capillaries, poor wound healing, and retarded growth in children. In patients with an inflammatory response, knowing the degree of the response is important in judging plasma micronutrient levels (Table 1) [Duncan A et al. Am J Clin Nutr. 2012].
Effect of Systemic Inflammation Response on Micronutrients
Use of multiple component additives, such as multi–trace element solution, in PN can produce deficiencies in some trace elements including copper, manganese, selenium, and zinc. Manganese deficiency is rare, while zinc deficiency is one of the more common micronutrient abnormalities and is a great concern in neonates. Toxicity due to excessive levels of some trace elements can occur during PN therapy. For example, manganese accumulates in the brain and can attain toxic levels. Toxicity has also been reported in high doses of zinc. Thus, it is wise to monitor patients during PN therapy for signs and symptoms of trace element deficiency and toxicity. To date, no evidence-based guidelines have been established concerning trace element monitoring during PN therapy.
Deficiency in L-carnitine is also a concern since this affects energy production by mitochondria. Deficiency is primarily genetic and secondarily due to low production or excessive loss in pregnancy, chronic kidney disease, malnutrition, or a diet high in protein or fat.
To conclude, there is a risk of micronutrient abnormalities in patients with long-term PN. If therapy continues for a year, this is almost assured. As a result, periodic monitoring is prudent.
- © 2015 SAGE Publications