Summary
Altered intestinal microbiota in critically ill patients leads to increased pathogens and infections, which may be improved with synbiotic therapy. Stress, antibiotics, and parenteral therapy have been linked to gut dysbiosis. Fecal microbiota transplantation is a promising treatment for Clostridium difficile infection and may work by altering bile acid composition.
- gut dysbiosis
- parenteral therapy
- enteral feeding
- fecal microbiota transplantation
- Clostridium difficile
- short-chain fatty acids
- epithelial barrier
- inflammation
- gut microbiota
The importance of the gut microbiota to health has been increasingly recognized as studies reveal the complex microbiota–host interactions taking place in the gastrointestinal (GI) tract. Gail A. Cresci, PhD, Cleveland Clinic, Cleveland, Ohio, USA, discussed the role of gut dysbiosis and its consequences in critically ill patients.
Many internal and external forces influence the gut microbiota. When a healthful diet including fermentable carbohydrate is ingested, gut bacteria produce short-chain fatty acids and other beneficial molecules. When crosstalk between the microbiota and the host breaks down, dysbiosis (microbial imbalance in the GI tract) and its clinical consequences can develop. Among factors that can alter the gut microbiota are host genetics, early colonization patterns, diet, stress, antibiotics, and other drugs.
Critically ill patients in the intensive care unit are often treated with antibiotics, artificial nutrition, opioids, and other substances that alter the intestinal environment. In such circumstances, the gut microbiota must compete for limited resources and survive in harsh conditions. Antibiotic effects on the microbiota include reduced total bacteria, changes in microbial composition, depleted lactobacilli, and impaired immune defenses [Willing BP et al. Nat Rev Microbiol. 2011]. Table 1 summarizes studies of gut microbiota alterations in critically ill patients, which demonstrate increased pathologic bacteria, virulence, and antibiotic resistance. These changes are associated with increased epithelial permeability, cell apoptosis, and altered mucus production, which are linked with multiple-organ dysfunction [Mittal R, Coopersmith CM. Trends Mol Med. 2014].
Studies of Gut Microbiota Alterations in Critically Ill Patients
Multiple studies have demonstrated some benefit with therapies using probiotics, prebiotics, or synbiotics for treating gut dysbiosis in critically ill patients, particularly with respect to reducing the occurrence of ventilator-associated pneumonia and other infectious complications [Shimizu K et al. Dig Dis Sci. 2013]. However, the studies have used different strains, concentrations, and delivery methods. Large studies with stringent inclusion and exclusion criteria are needed to determine the optimal strains for targeting depleted gut bacteria.
Daniel H. Teitelbaum, MD, University of Michigan, Ann Arbor, Michigan, USA, discussed the intestinal mucosal changes associated with parenteral nutrition (PN) that create an environment conducive to a shift in the gut microbiota and its translocation into the host. Dr Teitelbaum’s laboratory has shown, using a mice model, that mice receiving PN have significantly increased bacterial translocation and sepsis rates associated with loss of mucosal barrier function, villus atrophy, and gut-associated lymphoid tissue aberrations (P < .01) [Sun X et al. JPEN J Parenter Enteral Nutr. 2006].
According to Dr Teitelbaum, bacterial classification by pyrosequencing found decreased Firmicutes and expansion of Proteobacteria and Bacteroidetes in the colon and small bowel of a PN mouse model compared with control mice. At the genus level, expansion of Salmonella, Shigella, Proteus, and several Gram-negative organisms has been observed, as well as reduction of potentially beneficial bacteria [Miyasaka EA et al. J Immunol. 2013]. The interface between the host and gut flora is critical and is mediated by toll-like receptors (TLRs) on epithelial cells and immunocytes [Fukata M, Abreu MT. Biochem Soc Trans. 2007]. Marked upregulation of TLRs with PN activates a signaling pathway that leads to a marked upregulation of proinflammatory cytokines [Akira S, Takeda K. Nat Rev Immunol. 2004].
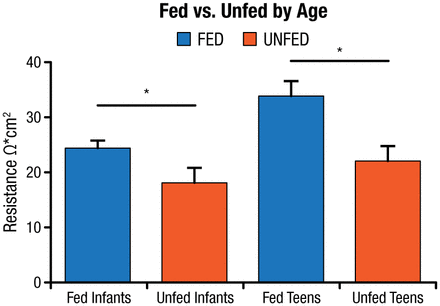
Dr Teitelbaum hypothesized that intestinal bacterial changes are a contributory factor to the mucosal inflammation observed in the small bowel with PN therapy. He tested this hypothesis in germ-free, enterally fed, and PN-fed mice and found decreased epithelial barrier function, increased inflammatory cytokines, and decreased T-regulatory cells in the PN mice versus the other groups. Based on these results, a study was conducted in clinical patients undergoing small bowel resection to determine if similar changes occur in humans [Ralls MW et al. JPEN J Parenter Enteral Nutr. 2014]. Samples were obtained from the mid–small bowel to the terminal ileum. Samples were considered unfed if the patient was without oral nutrition for > 14 days. Bacterial pyrosequencing revealed a difference in bacterial composition between the fed and unfed groups. Transepithelial resistance on samples from fed and unfed infants and adolescents found a significant decline in epithelial barrier function in the unfed patients (P < .05; Figure 1) [Ralls MW et al. Surgery. 2015].
Transepithelial Resistance: Decreased Epithelial Barrier Function in Unfed Patients
*P < .05 by t test.
Unfed, no oral nutrition for > 14 d.
Adapted from Surgery, Ralls MW et al. Enteral nutrient deprivation in patient leads to a loss of intestinal epithelial barrier function, Copyright 2015, with permission from Elsevier.
Dr Teitelbaum concluded that the same changes in bacterial composition, epithelial barrier function, and inflammation that were observed in mice also occur in humans. These studies show that the microbiome plays an important role in the adverse effects of PN.
Treatment with antibiotics, which alters gut microbiota, is a major risk factor for Clostridium difficile infection (CDI), a severe, fulminant, and often recurrent disease that is an important cause of infectious disease death in the United States. Michael J. Sadowsky, PhD, University of Minnesota, St Paul, Minnesota, USA, described fecal microbiota transplantation (FMT) for recurrent CDI.
In the past, fresh fecal slurries were used for FMT [Hamilton MJ et al. Gut Microbes. 2013]. Current methods use purified, standardized, frozen fecal microbiota preparations made by selective sieving, filtration, and differential centrifugation to obtain a highly pure microbe preparation. According to Dr Sadowsky, the University of Minnesota has established an FMT program with a rigorously screened donor volunteer program and frozen fecal microbe banking. High-throughput 16S amplicon sequencing has demonstrated that before FMT, CDI patient samples are dominated by Proteobacteria [Hamilton MJ et al. Gut Microbes. 2013]. Donor samples and patient samples taken after engraftment are dominated by Bacteroidetes and Firmicutes.
The success rate for CDI cure is 91% with the first FMT engraftment and 98% with 2 FMTs [Brandt L et al. Am J Gastroenterol. 2012]. A direct correlation between engraftment and the presence of secondary bile acids has been observed. Whereas primary bile acids promote C. difficile spore germination, secondary bile acids, which are produced by gut bacteria, inhibit spore germination and growth. A study to determine fecal bile acid composition before and after FMT in patients (n = 12) with recurrent CDI sequenced bacterial 16S rRNA genes and measured fecal bile acids [Weingarden AR et al. Am J Physiol Gastrointest Liver Physiol. 2014]. Eleven of the patients recovered fully after 1 FMT. Fecal primary bile acids decreased and secondary bile acids increased after FMT.
These investigations show that FMT is an alternative option to colectomy for some severe CDI cases. Two FMT procedures are recommended for severe CDI: one to stabilize the patient and one to ensure engraftment. Engraftment results in patient recovery and changes in bile acids. The changes in fecal bile acid composition may be a mechanism of FMT. Formal clinical trials of FMT are planned for the future.
- © 2015 SAGE Publications