Summary
Several large trials evaluating the efficacy of carotid endarterectomy (CEA) versus carotid artery stenting (CAS) are currently underway, with a large meta-analysis to include data from over 5000 patients planned for 2019. This article discusses the results of the ACST-2 [Halliday A et al. Eur J Vasc Endovasc Surg 2013], SPACE-2 [Reiff T et al. J Stroke 2009], and CREST-2 trials.
- Cerebrovascular Disease
- Interventional Techniques & Devices
- Cerebrovascular Disease
- Interventional Techniques & Devices
- Cardiology & Cardiovascular Medicine
Several large trials evaluating the efficacy of carotid endarterectomy (CEA) versus carotid artery stenting (CAS) are currently underway, with a large meta-analysis to include data from over 5000 patients planned for 2019. Alison Halliday, MD, University of Oxford, Oxford, United Kingdom, reflected on the results of the ACST-2 [Halliday A et al. Eur J Vasc Endovasc Surg 2013], SPACE-2 [Reiff T et al. J Stroke 2009], and CREST-2 trials.
Carotid stenosis can be attributed to ∼20% of ischemic strokes. Over the past 40 years, multiple clinical trials have evaluated the efficacy of CEA versus no intervention and CEA versus CAS. In the 1990s, the ACST-1 trial evaluated immediate versus deferred CEA, in which both physician and patients were substantially uncertain about the need for immediate CEA [Halliday A et al. Lancet 2004]. In the ACST-1 trial, the hazard of surgery was ∼3%, but the absolute risk of stroke was decreased by 6% over 10 years in both men and women. In 2010, the CREST trial randomized 1183 asymptomatic patients with carotid artery stenosis to undergo CAS or CEA [Brott TG et al. N Engl J Med 2010]. The primary endpoint of the trial was the composite of stroke, myocardial infarction, or death from any cause during the periprocedural period or any ipsilateral stroke within 4 years after randomization. There was no difference in the primary endpoint of the trial between CAS and CEA (7.2% vs 6.8%; HR, 1.11; 95% CI, 0.81 to 1.51; p=0.51).
It would be ideal to design a randomized controlled trial that evaluates the efficacy of CEA, CAS, and best medical treatment (BMT) simultaneously; however, the number of patients required is too large. Therefore, several trials are currently ongoing that will evaluate the best intervention for carotid artery stenosis separately.
The SPACE-2 study will have two sub-trials: SPACE-2A will evaluate CEA plus BMT versus BMT alone and SPACE-2B will evaluate CAS plus BMT versus BMT alone [Reiff T et al. J Stroke 2009]. Approximately 1636 patients will be enrolled and randomized in each sub-trial.
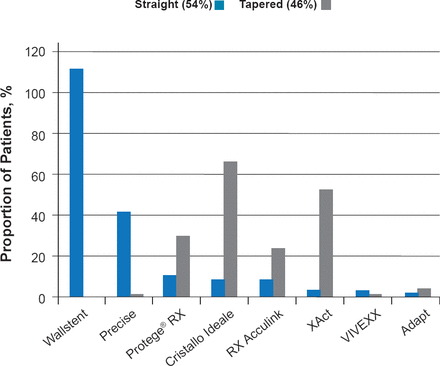
In 2010, the multicenter ACST-2 trial began enrollment to directly evaluate CEA versus CAS in asymptomatic patients with tight stenosis requiring intervention [Halliday A et al. Eur J Vasc Endovasc Surg 2013]. Enrollment reached 1287 in 2013, and the first 1000 patients had a median age of 71, with 96% of patients having 70% to 99% stenosis and 20% of patients having 70% to 100% contralateral stenosis. In addition, 30% of patients had diabetes, 11% renal failure, 6% atrial fibrillation, and 37% ischemic heart disease. In ACST-2, 93% of patients were receiving antiplatelet therapy, 89% antihypertensive therapy, and 85% lipid-lowering therapy at study entry. In the first 800 patients, 54% received a straight stent and 46% received a tapered stent (Figure 1). Preliminary results demonstrate that the rate of disabling and fatal stroke or myocardial infarction at ≤30 days is 1%, which is reduced from 1.7% in the previous ACST trial. By 2019, it is expected that enrollment for ACST-2 will reach ∼3000 patients, and a metaanalysis including CREST-2, SPACE-2, and ACST-2 is planned that will include > 5000 patients.
Stents Used in the First 800 Patients of ACST-2
Data from large trials that evaluate CEA versus CAS head-to-head is greatly anticipated and has the potential to provide a foundation for evidence-based medicine in the treatment of carotid artery stenosis.
- © 2013 MD Conference Express®