Summary
This article presents 1-year data from the Durable Polymer-Based Stent Challenge of Promus Element Versus Resolute Integrity in an All Comers Population [DUTCH PEERS (TWENTE II); von Birgelen C et al. Lancet 2013 (epublished ahead of print)] study. The results demonstrated comparable clinical outcomes with zotarolimus-eluting stents and everolimus-eluting stents (EES); both are third-generation, permanent, polymer-based, drug-eluting stents. There was no significant difference in efficacy and safety between the two stents, and longitudinal stent deformation was only seen in the EES group.
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Cardiology & Cardiovascular Medicine
- Cardiology Clinical Trials
Clemens von Birgelen, MD, PhD, Thoraxcentrum Twente, Enschede, The Netherlands, presented 1-year data from the Durable Polymer-Based Stent Challenge of Promus Element Versus Resolute Integrity in an All Comers Population [DUTCH PEERS (TWENTE II); von Birgelen C et al. Lancet 2013 (epublished ahead of print)] study. The results demonstrated comparable clinical outcomes with zotarolimus-eluting stents (ZES) and everolimus-eluting stents (EES); both are third-generation, permanent, polymer-based, drug-eluting stents (DES). There was no significant difference in efficacy and safety between the two stents, and longitudinal stent deformation was only seen in the EES group.
The introduction of DES has constituted a major breakthrough in the field of interventional cardiology, markedly reducing the incidence of restenosis and morbidity [Karjalainen P, Nammas W. Minerva Cardioangiol 2011]. Newer third-generation stent technology has been developed in an attempt to further enhance DES performance, and these durable-polymer-based DES aim to meet the need for more flexible and highly deliverable devices for the treatment of more challenging coronary lesions and vascular anatomy. While the coatings of third-generation stents remain similar to those of second-generation DES, changes in the material and design of their more flexible bare-metal platforms have the potential to reduce longitudinal stent stability.
To date, however, long-term data to compare the efficacy of currently available third-generation DES are still lacking [Akin I et al. Herz 2011].
DUTCH PEERS is a prospective, single-blinded, randomized, controlled trial in patients requiring percutaneous coronary intervention (PCI) with DES placement. The study was performed in 4 PCI centers in The Netherlands, and was designed to evaluate clinical outcomes after stenting with two third-generation DES that are frequently used clinically, but that had not previously been compared. It is the first all-comer trial with the platinum-chromium EES to be undertaken in a predominantly Caucasian population. A total of 20.4% of patients presented with a STEMI (requiring primary PCI), overall 58.6% had acute coronary syndromes, and 59.0% were treated for at lesions in small vessels.
Patients were eligible to be included if they were aged ≥18 years, were able to provide informed consent, and had coronary artery disease and lesions eligible for DES treatment. There were no restrictions on clinical presentation or extent of coronary artery disease. Exclusion criteria included participation in another randomized clinical trial prior to reaching the primary endpoint; planned surgery within 6 months of PCI unless dual antiplatelet therapy was able to be continued during the surgery; pregnancy; life expectancy <1 year; and P2Y12 receptor antagonist intolerance, resulting in inability to adhere to dual antiplatelet therapy, or intolerance to heparin, aspirin, or components of the DES.
The primary endpoint was target vessel failure (TVF) at 1 year and was defined as the composite of cardiac death, target vessel-related myocardial infarction, or target vessel revascularization.
A total of 1811 patients with 2371 target lesions were enrolled in the study and randomized to either treatment with third-generation cobalt-chromium ZES (n=906; 1205 lesions), or platinum-chromium EES (n=905; 1166 lesions).
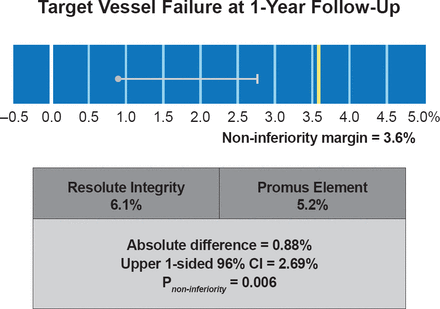
At 1 year, there was no statistically significant difference between the ZES and EES groups on the primary composite endpoint of TVF (6.1% vs 5.2%; p=0.42; p=0.006 for noninferiority; Figure 1), or in its individual components. Similarly, there was no significant difference between the two groups in the incidence of definite or probable stent thrombosis (0.6% vs 0.9%; p=0.4). There were no definite stent thromboses recorded at 3 months post implantation in either group. Longitudinal stent deformation was seen only in the EES group (1.0%; p=0.002) but not related to any adverse clinical events.
Primary Endpoint Data for DUTCH PEERS (TWENTE II)
Reproduced with permission from C von Birgelen, MD.
Prof. von Birgelen concluded that data from the DUTCH PEERS study demonstrate that treatment with either third-generation ZES or EES result in similar, clinical outcomes at 1 year.
- © 2013 MD Conference Express®