Summary
The current recommended treatment for patients with coronary bifurcation lesions is main branch stenting with provisional side branch stenting. This approach can lead to suboptimal results in the side branch of true bifurcation lesions, in which disease affects the origin of both branches. The objective of the Prospective Single Blind, Randomized Controlled Study to Evaluate the Safety & Effectiveness of the Tryton Side Branch Stent Used With DES in Treatment of de Novo Bifurcation Lesions in the Main Branch & Side Branch in Native Coronaries [TRYTON; NCT01258972] was to compare clinical and angiographic outcomes of the provisional one-stent strategy with the Tryton bifurcation two-stent approach in patients with true bifurcation lesions.
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
- Interventional Techniques & Devices
- Cardiology Clinical Trials
The current recommended treatment for patients with coronary bifurcation lesions is main branch stenting with provisional side branch stenting. This approach can lead to suboptimal results in the side branch of true bifurcation lesions, in which disease affects the origin of both branches. The objective of the Prospective Single Blind, Randomized Controlled Study to Evaluate the Safety & Effectiveness of the Tryton Side Branch Stent Used With DES in Treatment of de Novo Bifurcation Lesions in the Main Branch & Side Branch in Native Coronaries [TRYTON; NCT01258972] was to compare clinical and angiographic outcomes of the provisional one-stent strategy with the Tryton bifurcation two-stent approach in patients with true bifurcation lesions. Martin B. Leon, MD, Columbia University Medical Center, New York, New York, USA, presented the results of this study
The Tryton stent is a cobalt alloy bare-metal stent. It is inserted in the proximal main vessel extending into the side branch, securing and protecting the side branch. A drug-eluting stent (DES) is placed in the main vessel through the Tryton stent. Finally, postdilation with a kissing balloon is performed to ensure complete lesion and ostium coverage of the side branch.
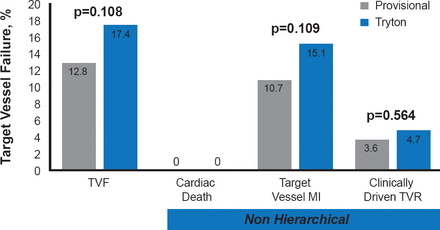
In the TRYTON study, 704 patients with true bifurcation lesions were randomized to treatment with the Tryton side branch stent and a DES main vessel stent (n=355) or a DES main vessel stent and provisional side branch stent (n=349). The trial was designed as a noninferiority trial with noninferiority margin of 5.5%. The primary endpoint (noninferiority) was target vessel failure (TVF) at 9 months, which was defined as a composite of cardiac death, periprocedural target vessel myocardial infarction (MI; defined as a creatine kinase [CK]-MB >3x upper limit of normal), or target vessel revascularization (TVR). The secondary endpoint (superiority) was the percent diameter stenosis (%DS) of the side branch at 9 months in the cohort who underwent followup angiography.
Patient demographics and clinical characteristics were similar between the two treatment groups. The Tryton stent was successfully implanted in 96.1% of patients in the Tryton group and 0.6% in the provisional group. Additional side branch stents were placed in 2.9% of the Tryton group and 8.0% of the provisional group.
While the rate of TVF was numerically higher in the Tryton arm than the provisional arm, the difference did not achieve statistical significance (17.4% vs 12.8%; p=0.108; Figure 1). The difference in the incidence of the primary endpoint was 4.6% between the two arms and the primary noninferiority margin was not met (upper 1-sided 95% CI, 10.3%; p=0.42 for noninferiority). Analysis of the components of the primary endpoint showed no statistically significant differences between the two arms. There were no cardiac deaths in either arm and >90% of the target vessel MIs were periprocedural.
Primary Endpoint: Target Vessel Failure and Components at 9 Months
MI=myocardial infarction; TVF=target vessel failure; TVR=target vessel revascularization.
Angiography showed that the secondary endpoint of side branch in-segment %DS was significantly lower in the Tryton arm (31.6%) compared with the provisional arm (38.6%; p=0.002; Table 1). The side branch in-segment minimal luminal diameter was significantly higher in the Tryton arm (1.56 mm) versus the provisional arm (1.36 mm; p<0.001). Angiography results for the main vessel showed no significant differences between the groups. Stent thrombosis was rare, with an overall rate of 0.4% (0.6% in the Tryton arm vs 0.3% in the provisional arm; p=1.00). There were no significant differences in restenosis rates between the two groups.
Angiographic Results at 9 Months
In this study, the Tryton two-stent strategy, when compared with a strategy of provisional stenting, did not meet the noninferiority clinical endpoint. This was largely due to a higher rate of small periprocedural CK-MB elevations in the patients treated with the Tryton stent; however, in side branches >2.25 mm, a Tryton two-stent strategy resulted in better angiographic results in the cohort of patients who underwent follow-up angiography.
- © 2013 MD Conference Express®