Summary
First-generation drug-eluting stents have reduced the risk of restenosis compared with bare-metal stents; however, these stents may have increased risk of stent thrombosis. Newer generation drug-eluting stents, which are constructed with biocompatible or biodegradable polymers, may have greater efficacy, safety, and device performance. The Randomized Clinical Comparison of Biomatrix Flex and Resolute Integrity trial [SORT-OUT VI; NCT01956448] compared the efficacy and safety of a zotarolimus-eluting stent with a biolimus-eluting stent in a population-based setting.
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Cardiology & Cardiovascular Medicine
- Cardiology Clinical Trials
- Interventional Techniques & Devices
First-generation drug-eluting stents have reduced the risk of restenosis compared with bare-metal stents; however, these stents may have increased risk of stent thrombosis. Newer generation drug-eluting stents, which are constructed with biocompatible or biodegradable polymers, may have greater efficacy, safety, and device performance. The Randomized Clinical Comparison of Biomatrix Flex and Resolute Integrity trial [SORT-OUT VI; NCT01956448], presented by Bent Raungaard, MD, Aalborg University Hospital, Aalborg, Denmark, compared the efficacy and safety of a zotarolimus-eluting stent with a biolimus-eluting stent in a population-based setting.
SORT-OUT VI was a prospective randomized, all-comers study designed to reflect clinical practice. A total of 2999 patients were randomized to receive either a zotarolimus-eluting permanent polymer stent (n=1502) or a biolimus-eluting biodegradable stent (n=1497). To be considered for the trial, patients had to have either stable coronary artery disease or acute coronary syndromes with ≥1 coronary lesion with a >50% diameter stenosis in a vessel with a reference diameter of 2.25 to 4.0 mm. Patients were excluded if they had a life expectancy <1 year, were allergic to aspirin, clopidogrel, prasugrel, ticagrelor, zotarolimus, or biolimus, or were not candidates for 12 months of dual antiplatelet treatment. The primary endpoint was a composite of major adverse cardiac events (MACE) defined as cardiac death, myocardial infarction (MI) or target lesion revascularization (TLR) at 12 months. Patient-driven clinical event detection was used, with data accessed from the Danish Civil Registration System, National Patient Registry, and Western Denmark Heart Registry.
Most baseline patient characteristics were well balanced between the two groups. The mean subject age was 65.8 years and 76% of the patients were men. More patients receiving the biolimus-eluting stent had undergone previous percutaneous intervention (PCI; 22.0% vs 18.7%; p=0.03). In the zotarolimus-eluting stent group, more patients had >1 lesion (25.3% vs 22.1%; p=0.04) and the total stent length per patient was longer (21.0 vs 18.0 mm; p<0.01).
At 12 months, the primary endpoint of MACE had occurred in 5.3% of patients with a zotarolimus-eluting stent compared with 5.1% of those with a biolimus-eluting stent (difference, 0.2%; upper one-sided 95% CI, 1.8%; noninferiority p=0.006).
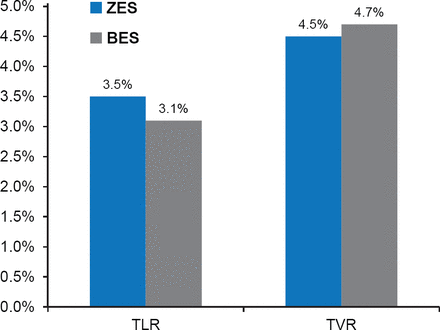
At 12 months, cardiac death had occurred in 1.5% of the zotarolimus-eluting stent group compared with 1.7% of the biolimus-eluting stent group (HR, 0.85; 95% CI, 0.48 to 1.50; p=0.58). MI was reported in 1.3% of patients in the zotarolimus-eluting stent group compared with 0.9% of patients in the biolimus-eluting stent group (HR, 1.43; 95% CI, 0.72 to 2.84; p=0.30). TLR was required in 3.5% of patients with the zotarolimus-eluting stent and 3.1% of biolimus-eluting stent (HR, 1.11; 95% CI, 0.75 to 1.65; p=0.80; Figure 1), while target vessel revascularization was required in 4.5% of patients with the zotarolimus-eluting stent and 4.7% of those with the biolimus-eluting stent (HR, 0.95; 95% CI, 0.68 to 1.32; p=0.75; Figure 1).
Target Lesion and Target Vessel Revascularization
BES=biolimus-eluting stent; TLR=target lesion revascularization; TVR=target vessel revascularization; ZES=zotarolimus-eluting stent.
Definite stent thrombosis was reported in 0.6% of patients with the zotarolimus-eluting stent compared with 0.4% of patients with the biolimus-eluting stent (HR, 1.29; 95% CI, 0.48 to 3.47; p=0.61). Definite or probable stent thrombosis occurred in 0.8% of patients with the zotarolimus-eluting stent compared with 0.5% of patients with the biolimus-eluting stent (HR, 1.73; 95% CI, 0.68 to 4.38; p=0.25).
The results of the SORT-OUT VI trial demonstrate that both zotarolimus-eluting stents and biolimus-eluting stents are associated with similar rates of cardiac death, MI, or TLR. The zotarolimus-eluting stent met the criteria for noninferiority compared to the biolimus-eluting stent in patients treated with PCI.
- © 2013 MD Conference Express®