Summary
Transcatheter aortic valve replacement (TAVR) is less invasive than surgical aortic valve replacement for patients with degenerative aortic stenosis who have a high risk of surgical complications. The purpose of the Safety and Efficacy Study of the Medtronic CoreValve System in the Treatment of Symptomatic Severe Aortic Stenosis in High Risk and Very High Risk Subjects Who Need Aortic Valve Replacement [CoreValve Extreme Risk; NCT01240902] was to evaluate the safety and efficacy of the CoreValve transcatheter heart valve for the treatment of symptomatic severe aortic stenosis in patients with a =50% risk of operative mortality or serious, irreversible morbidity at 30 days.
- Cardiology Clinical Trials
- Valvular Disease
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
- Valvular Disease
- Interventional Techniques & Devices
Transcatheter aortic valve replacement (TAVR) is less invasive than surgical aortic valve replacement for patients with degenerative aortic stenosis who have a high risk of surgical complications. The purpose of the Safety and Efficacy Study of the Medtronic CoreValve System in the Treatment of Symptomatic Severe Aortic Stenosis in High Risk and Very High Risk Subjects Who Need Aortic Valve Replacement [CoreValve Extreme Risk; NCT01240902], presented by Jeffrey J. Popma, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA, was to evaluate the safety and efficacy of the CoreValve transcatheter heart valve for the treatment of symptomatic severe aortic stenosis in patients with a ≥50% risk of operative mortality or serious, irreversible morbidity at 30 days.
Patients at high risk of surgical complications in whom an 18 French vascular access sheath could be placed into the iliofemoral vessel were randomized to treatment with a CoreValve by iliofemoral (n=487) or noniliofemoral (n=147) access. Patients included in the trial had severe aortic stenosis, defined as aortic valve area (AVA) ≤0.8 cm2 or AVA index ≤0.5 cm2/m2; a mean gradient >40 mm Hg or peak velocity >4 m/second at rest or with dobutamine stress (if left ventricular ejection fraction was <50%); and NYHA Functional Class II or higher. The primary endpoint was all-cause mortality or major stroke at 12 months. Clinical and echocardiographic assessments were performed at baseline (n=471), 1 month (n=435), and 1 year (n=355). There was no control arm in the trial.
The primary analysis was performed in the as-treated population (n=471). The patients were elderly (aged 83.1±8.6 years), 49% were men, and 91.9% had severe symptoms (NYHA Class III or IV). The composite rate of all-cause mortality and major stroke was 9.3% (95% CI, 6.7 to 12.0) at 1 month and 25.5% (95% CI, 21.6 to 29.4; p<0.0001) at 1 year. Variables predictive for the primary endpoint were Society of Thoracic Surgeons (STS) score >15 (p=0.02), coronary artery disease (p=0.003), and assisted living (p<0.001). The primary endpoints of all-cause and cardiovascular mortality rates at 1 year were 24.0% and 17.9%, respectively. The major stroke rate was 4.1% at 1 year.
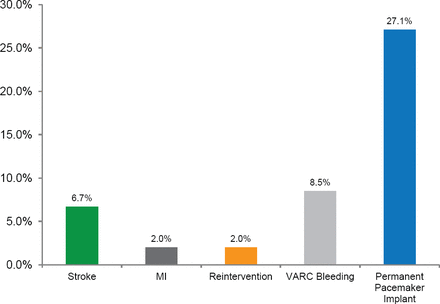
The incidence of major secondary endpoint rates were assessed at 1 year (Figure 1). Of the patients alive at 1 year, 90% of patients had improvement of ≥1 NYHA class and 60% of patients improved by ≥2 NYHA classes. Echocardiography showed that effective orifice area increased from 0.73 cm2 at baseline to 1.82 cm2 at discharge and to 1.89 cm2 at 1 year. The mean gradient decreased from 47.4 mm Hg at baseline to 9.4 mm Hg at discharge and to 8.8 mm Hg at 1 year.
Major Secondary Endpoints
MI=myocardial infarction; VARC=Valve Academic Research Consortium.
At 1 month, moderate paravalvular leak was present in 41.6% of patients and severe paravalvular leak was present in 11.0%. At 1 year, 28.8% of patients had moderate paravalvular leak and 4.1% had severe paravalvular leak. The severity of paravalvular leak declined in the majority of patients with moderate paravalvular leak at 1 month who survived to 1 year (80%). Patients with severe paravalvular leak had higher mortality rates when compared with patients without paravalvular leak (17.9% vs 85.7%; p<0.001).
The CoreValve Extreme Risk study evaluated iliofemoral implantation of the CoreValve prosthesis in patients at extreme risk for mortality and morbidity from surgery. At 1 year after implantation, 25% of patients had either died or had a stroke. The rates of moderate and severe aortic regurgitation were low and improved over time. Patients with severe paravalvular leak had higher mortality compared with patients with no paravalvular leak. These results provide evidence for the safety and efficacy of TAVR with the CoreValve in patients at high risk of surgical complications.
- © 2013 MD Conference Express®