Summary
Warfarin is widely used for stroke prevention in patients with atrial fibrillation (AF) but novel oral anticoagulants have been developed that may be just as effective [Dogliotti A et al. Clin Cardiol 2013]. Edoxaban is a direct oral factor Xa inhibitor administered once daily with a rapid onset of action. Warfarin and two doses of edoxaban were compared in the Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation — Thrombolysis in Myocardial Infarction 48 study [ENGAGE AF-TIMI 48; Giugliano RP et al. N Engl J Med 2013].
- Cardiology Clinical Trials
- Arrhythmias
- Cerebrovascular Disease
- Cardiology Clinical Trials
- Arrhythmias
- Cerebrovascular Disease
- Cardiology
Warfarin is widely used for stroke prevention in patients with atrial fibrillation (AF) but novel oral anticoagulants have been developed that may be just as effective [Dogliotti A et al. Clin Cardiol 2013]. Edoxaban is a direct oral factor Xa inhibitor administered once daily with a rapid onset of action. Warfarin and two doses of edoxaban were compared in the Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation - Thrombolysis in Myocardial Infarction 48 study [ENGAGE AF-TIMI 48; Giugliano RP et al. N Engl J Med 2013]. This trial enrolled 21,105 patients with AF at moderate to high risk of stroke (CH A DS2 ≥2) at 1393 centers in 46 countries. Patients were randomized in a double-blind, double-dummy manner to one of three regimens: 1) warfarin to an international normalized ratio (INR) of 2.0 to 3.0 (n=7036); 2) edoxaban 60 mg/day (high dose; n=7035); or 3) edoxaban 30 mg/day (low dose; n=7034). Edoxaban doses were decreased by 50% if patients had a creatinine clearance of 30 to 50 mL/minute, had a body weight ≤60 kg, or were taking a strong P-glycoprotein inhibitor. The primary endpoint was a composite of stroke or systemic embolic events (SEE). The primary analysis was a noninferiority comparison performed in those patients who took at least one dose of study medications (modified intention-to-treat [mITT] population) during the time that patients were treated (on-treatment time period). Secondary analyses evaluated all patients randomized during the overall treatment period (ITT).
Robert P. Giugliano, MD, Brigham and Women's Hospital, Boston, Massachusetts, USA, presented the primary results from the ENGAGE AF-TIMI 48 trial. Demographic characteristics were well-balanced with no differences between treatment groups in any variable. The median participant age was 72 years (interquartile range, 64 to 78 years), 38% were female, and the mean CHADS2 score was 2.8±1.0. In terms of medical history, 94% had hypertension, 57% had prior congestive heart failure, 36% had diabetes mellitus, and 28% had a prior stroke or transient ischemic attack. A quarter of the patients had an edoxaban dose reduction at randomization.
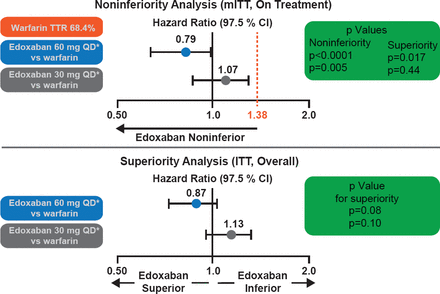
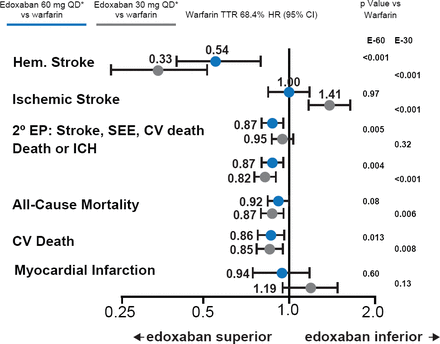
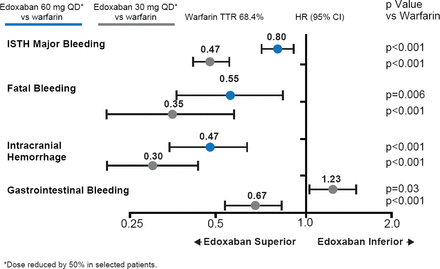
Over 99% of the patients completed the trial, and only one patient was lost to follow-up. Overall the median time in the therapeutic range was 68.4% (interquartile range, 56.5 to 77.4). The primary endpoint was based on a median follow-up of 2.8 years. Both doses of edoxaban met the noninferiority criteria (p<0.0001 for high-dose edoxaban and p=0.005 for low-dose; Figure 1) in the mITT population while on treatment. Neither edoxaban regimen was statistically superior for the primary endpoint (p=0.08 for high dose and p=0.10 for low dose in the ITT analysis during the overall time period; Figure 1). Both doses of edoxaban had significant reductions in key secondary outcomes (Figure 2) and safety endpoints (Figure 3).
Primary Endpoint Results
Both dose regimens of edoxaban were noninferior to warfarin in the primary noninferiority analysis. The high-dose regimen tended to be more effective at reducing stroke/SEE compared with warfarin and the low-dose regimen less effective.
Reproduced with permission from RP Giugliano, MD.
Secondary Endpoint Results
Both dose regimens of edoxaban reduced hemorrhagic stroke, death or ICH, and CV death compared with warfarin. The low-dose regimen was not as effective as warfarin at reducing ischemic stroke. CV=cardiovascular; E-60=edoxaban 60 mg QD dose group; E-30=edoxaban 30 mg QD dose group; ICH=intracerebral hemorrhage; SEE=systemic embolic events, TTR=time in therapeutic range.
Reproduced with permission from RP Giugliano, MD.
Key Safety Results
*Dose reduced by 50% in selected patients. Both dose regimens of edoxaban substantially reduced major, fatal, and intracranial bleeding. Gastrointestinal bleeding was increased with high-dose edoxaban compared with warfarin, but reduced with the low-dose regimen compared with warfarin. TTR=time in therapeutic range.
Reproduced with permission from RP Giugliano, MD.
Both edoxaban regimens were well tolerated and no significant differences were observed in serious adverse events or liver abnormalities compared with warfarin. In terms of net clinical outcomes, both the high-dose and low-dose edoxaban regimens led to significant reductions in composite endpoints of stroke/SEE/death/major bleeding (p=0.003 and p<0.001, respectively), disabling stroke/life-threatening bleeding/death (p=0.008 and p<0.001, respectively), and stroke/SEE/life-threatening bleeding/death (p=0.003 and p=0.007, respectively).
In this large, randomized, controlled international trial, once-daily edoxaban was noninferior to well-managed warfarin for the prevention of stroke and SEE, with a trend toward fewer stroke/SEEs observed with the higher dose. Both edoxaban regimens had superior net clinical outcomes, which assessed various combinations of death, stroke, and bleeding events, compared with warfarin.
- © 2013 MD Conference Express®