Summary
Warfarin is a widely used medication with a very narrow therapeutic window. Limited evidence suggests that utilizing pharmacogenetic information on top of clinical information could improve warfarin dosing, but large, well-conducted studies are lacking. This article discusses the key results of the Clarification of Optimal Anticoagulation Through Genetics trial [COAG; Kimmel SE et al. N Engl J Med 2013], which compared warfarin initiation using a clinical algorithm with or without the addition of pharmacogenetic information.
- Cardiology Genomics
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
- Cardiology Genomics
- Cardiology Clinical Trials
Warfarin is a widely used medication with a very narrow therapeutic window. Limited evidence suggests that utilizing pharmacogenetic information on top of clinical information could improve warfarin dosing, but large, well-conducted studies are lacking. Stephen E. Kimmel, MD, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA, presented the key results of the Clarification of Optimal Anticoagulation Through Genetics trial [COAG; Kimmel SE et al. N Engl J Med 2013]. COAG was a large, randomized double-blind trial conducted at 18 centers in the United States. The study compared warfarin initiation using a clinical algorithm with or without the addition of pharmacogenetic information. The analysis was performed in all randomized patients as well as for those in whom a significant difference in the initial warfarin dose was predicted between the algorithms.
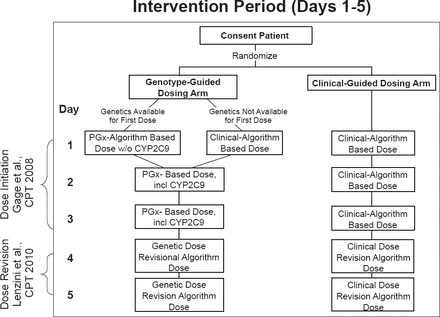
A total of 1015 patients were randomized to the clinical-guided arm (n=501) or the pharmacogenetic-guided (PG) arm (n=514). Genotype information on cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase complex 1 (VKORC1) was available for ≥99% of subjects in each arm. Participants were stratified by clinic center and self-reported race (black vs nonblack). Stratification by race was performed because of a priori knowledge that genotype-guided algorithms do not perform as well in black patients. Clinical variables used to guide warfarin initiation included age, race, body surface area, smoking status, amiodarone use, target international normalized ratio (INR), and indication for warfarin use. A dose-revision algorithm used on Days 4 and/or 5 was used for dose adjustments (Figure 1). Clinical variables in this algorithm included age, race, body surface area, diabetes, stroke, amiodarone use, fluvastatin use, target INR, natural log INR, and prior warfarin doses used. The primary endpoint was the percentage of time in the therapeutic range (TTR) during the first 28 days of warfarin treatment.
Intervention Period (Days 1 to 5)
Reproduced from Kimmel SE et al. Rationale and design of the Clarification of Optimal Anticoagulation through Genetics trial. Am Heart J 2013;166(3)435–441. With permission from Elsevier.
Patient demographic and clinical characteristics were similar between the two arms. Approximately two thirds were started on warfarin as an inpatient. Fifty-eight percent of patients were taking warfarin for deep vein thrombosis or pulmonary embolism only, and 22% were taking warfarin for atrial fibrillation/flutter only. Genotypes were well balanced between the groups and the prevalence was as expected.
The mean TTR for the PG arm was 45.2% (SD 26.6) compared with 45.4 (SD 25.8) in the clinical-guided arm after 4 weeks of therapy (mean difference, −0.2; 95% CI, −3.4 to 3.1; p=0.91). The coprimary analysis of the TTR was conducted in those who had a ≥1.0 mg/day difference in starting dose by the two algorithms; this analysis was consistent with the primary results. Race was a highly significant interaction: black patients in the PG group had a lower TTR than nonblacks (mean difference, −8.3; 95% CI, −15 to −2.0; p=0.01). There were no significant differences between treatment groups in any safety endpoint.
In this large randomized trial, initiating warfarin therapy by adding genotype information to a clinical-guided algorithm did not improve anticoagulation control during the first 4 weeks. The clinical-guided algorithm appeared be a more appropriate choice for black patients. Dr. Kimmel concluded that the COAG trial highlights the importance of performing randomized trials for pharmacogenetics, particularly for complex medicine regimens such as warfarin.
- © 2013 MD Conference Express®