Summary
In adults with heart failure and preserved ejection fraction, the mineralocorticoid receptor antagonist spironolactone did not significantly reduce the composite primary outcome of cardiovascular mortality, aborted cardiac arrest, or hospitalization for heart failure (HF) compared with placebo, but it did reduce HF hospitalizations. This article presents the results of the Treatment of Preserved Cardiac Function With an Aldosterone Antagonist [TOPCAT; NCT00094302] study.
- Cardiology Clinical Trials
- Heart Failure
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
- Heart Failure
In adults with heart failure and preserved ejection fraction (HFpEF), the mineralocorticoid receptor antagonist spironolactone did not significantly reduce the composite primary outcome of cardiovascular (CV) mortality, aborted cardiac arrest, or hospitalization for heart failure (HF) compared with placebo, but it did reduce HF hospitalizations (Table 1). Marc A. Pfeffer, MD, PhD, Brigham and Women's Hospital, Boston, Massachusetts, USA, presented the results of the Treatment of Preserved Cardiac Function With an Aldosterone Antagonist [TOPCAT; NCT00094302] study.
Results for Primary Outcome and Its Components
The hypothesis for the benefit of aldosterone antagonism in HFpEF patients was based upon mechanistic data in combination with the benefits observed in outcomes trials of patients with heart failure and reduced ejection fraction (HFrEF) as well as in the post-myocardial infarction (MI) setting. These included the RALES [Pitt B et al. N Engl J Med 1999], EMPHASIS [Zannad F et al. N Engl J Med 2011], and the EPHESUS studies [Pitt B et al. N Engl J Med 2003].
The National Heart, Lung and Blood Institute-funded, international, multicenter, double-blind, placebo-controlled TOPCAT study randomized 3445 patients with symptomatic HF (NYHA II to IV), left ventricular ejection fraction (LVEF) ≥45%, and either HF hospitalization within 1 year prior to randomization or elevated natriuretic peptide levels (BNP ≥100 pg/mL or NT-proBNP ≥360 pg/mL) within 60 days prior to randomization. Stratification based on hospitalization in the past year for HF management was performed [Desai A et al. Am Heart J 2011]. Patients were started on spironolactone 15 mg/placebo with titration to 30 mg at 4 weeks if there were no tolerability concerns; further titration to 45 mg daily was based on investigator discretion. At 8 months, the mean spironolactone dose was 25 mg. The mean follow-up was 3.3 years. Discontinuation of the study drug increased each year, with 34.3% of spironolactone patients and 31.4% of placebo patients discontinuing by 3 years. Vital status was unknown for 67 spironolactone patients (3.9%) and 65 placebo patients (3.8%).
Within each stratum, 71.5% were hospitalized within the prior year for HF and 28.5% had elevated natriuretic peptides. Baseline characteristics included a median age of 69 years and 52% were women. The median LVEF was 56%. NYHA II was present in 63% and NYHA III in 33% patients. Pertinent baseline findings included history of MI (26%), diabetes mellitus (33%), median systolic blood pressure 130 mm Hg, and eGFR <60 mL/min/1.73 m2 (39%).
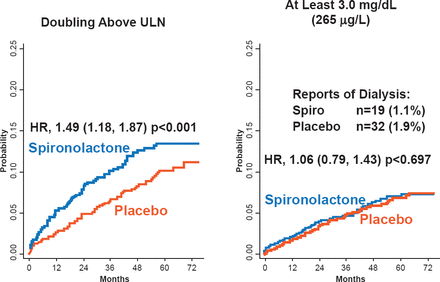
No overall differences were found in the rate of serious adverse events (48.5% spironolactone vs 49.6% placebo). Significantly more patients in the spironolactone group had hyperkalemia (≥5.5 mmol/L; 18.7% vs 9.1%; p<0.001) but fewer had hypokalemia compared with placebo (≤3.5 mmol/L; 16.2% vs 22.9%; p<0.001). In addition, the spironolactone group had a significantly increased risk of elevated creatinine (2x upper limit of normal). However, the percentage of patients requiring dialysis or having a creatinine level of at least 3.0 mg/dL was similar between the groups (Figure 1). Dr. Pfeffer stated that the use of spironolactone in patients with HFpEF requires careful monitoring of potassium and creatinine.
The Effect of Spironolactone on Creatinine and Risk of Dialysis
ULN=upper limit of normal.
Reproduced with permission from MA Pfeffer, MD, PhD.
The overall trial results were consistent across 21 of 22 prespecified subgroups, except in patients with elevated natriuretic peptides who demonstrated a significant reduction in the primary endpoint with spironolactone (HR, 0.65; 95% CI, 0.49 to 0.87; p=0.003). An exploratory post hoc analysis also revealed a significant geographic variation in the placebo event rates and the reduction of the primary endpoint (p=0.122). The primary outcome occurred in 31.8% of the placebo patients in the United States, Canada, Argentina, and Brazil; in these countries, spironolactone was associated with a HR of 0.82 (95% CI, 0.69 to 0.98). In Russia and the Republic of Georgia, the primary outcomes occurred in 8.4%; in these countries, spironolactone was not associated with better outcomes (HR, 1.10; 95% CI, 0.79 to 1.51). Physician judgment should guide the decision whether to use spironolactone to reduce HF hospitalization in a specific patient. However, these data do not support the broad use of spironolactone in patients with HFpEF to reduce CV events.
- © 2013 MD Conference Express®