Summary
In the Renal Optimization Strategies Evaluation in Acute Heart Failure Study [ROSE AHF; NCT01132846] treatment with low-dose dopamine or low-dose nesiritide did not improve renal dysfunction compared with placebo. The results were part of a National Heart, Lung, and Blood Institute-funded study.
- Cardiology Clinical Trials
- Heart Failure
- Renal Disease
- Cardiology Clinical Trials
- Heart Failure
- Cardiology & Cardiovascular Medicine
- Renal Disease
In the Renal Optimization Strategies Evaluation in Acute Heart Failure Study [ROSE AHF; NCT01132846] treatment with low-dose dopamine or low-dose nesiritide did not improve renal dysfunction compared with placebo. The results of the National Heart, Lung, and Blood Institute -funded study were presented by Horng H. Chen, MD, Mayo Clinic, Rochester, Minnesota, USA.
ROSE-AHF examined whether the addition of low-dose dopamine (2 μg/kg/min) or low-dose nesiritide (0.005 μg/kg/min without bolus) to diuretic therapy would enhance decongestion and preserve renal function when compared with placebo in patients with acute heart failure (AHF) and ≥1 symptom (dyspnea, orthopnea, edema) or ≥1 sign (rales, edema, ascites, chest x-ray), and an estimated glomerular filtration rate (eGFR) 15 to 60 mL/min/1.73 m2. For the first 24 hours, all patients received standardized diuretic dosing (2.5-times the outpatient dose) and patients were enrolled within 24 hours of hospitalization.
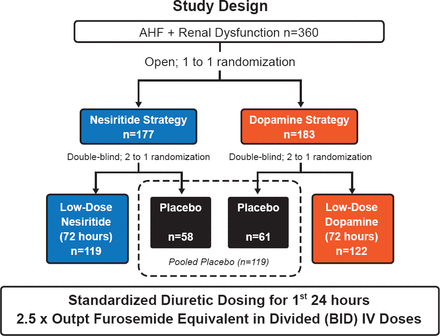
The randomization schema and number of patients in each group are shown in Figure 1. The two coprimary endpoints were cumulative urinary volume from randomization through 72 hours (decongestion endpoint), and change in serum cystatin-C concentration from randomization to 72 hours (renal function endpoint).
ROSE AHF Study Design
Reproduced with permission from H Chen, MD.
Patients randomized had a median age of 70 years, 73% were male, and 26% had an ejection fraction (EF) >50%. Over half of patients (67%) had been hospitalized for AHF in the prior year. Their median eGFR was 44.5 mL/min/1.73 m2, NT-proBNP was 4972 pg/mL, and the median outpatient dose of furosemide was 80 mg/day.
Results for the dopamine strategy showed no significant difference between active treatment and placebo in 72-hour urine volume (8.5 vs 8.3 L, respectively; p=0.58), or cystatin-C concentration (0.12 vs 0.11 mg/L; p=0.72). The lack of effect was consistent across prespecified subgroups, except for patients with preserved EF (>50%) who tended to have lower urine volume with dopamine compared with placebo (p=0.01).
No significant treatment effect was seen with dopamine on secondary endpoints related to decongestion, renal function, or symptom relief. There was less study drug dose reduction or discontinuation due to hypotension in the dopamine group, but they were more likely to have study drug dose reduction or discontinuation due to tachycardia. The overall incidence of study drug discontinuation before 72 hours due to any cause was similar between the two groups. As for clinical outcomes, the composite of 60-day death, unscheduled visits, or HF readmission was similar between the two groups (HR, 1.15; 95% CI, 0.74 to 1.78; p=0.53), as was the rate of 180-day mortality (HR, 0.95; 95% CI, 0.54 to 1.68; p=0.87).
In the nesiritide group, there was no significant difference between active treatment and placebo in 72-hour urine volume (8.6 vs 8.3 L, respectively; p=0.25), or cystatin-C concentration (0.07 vs 0.11 mg/L, respectively; p=0.35). The lack of benefit was consistent across prespecified subgroups. There was a nonsignificant trend suggesting a differential effect in patients with reduced EF compared with patients with preserved EF. Patients with reduced EF who received nesiritide tended to have greater urine output volume (p=0.06) and less change in cystatin-C concentration when compared with patients receiving placebo (p=0.09). There was no significant treatment effect on secondary endpoints related to decongestion, renal function, or symptom relief. Patients receiving nesiritide had rates of study drug dose reduction or discontinuation due to hypotension that were numerically higher than in patients receiving placebo (18.8% vs 10.4%; p=0.07). The overall incidence of study drug discontinuation before 72 hours for any reason was similar in both the treatment and placebo group (25% vs 25%; p=0.94). The rate of 180-day mortality was similar between the groups (HR, 0.91; 95% CI, 0.51 to 1.61; p=0.74). The composite rate of 60-day death, unscheduled visits, or HF readmission, however, showed a nonsignificant trend favoring nesiritide (HR, 0.71; 95% CI, 0.44 to 1.15; p=0.16).
In patients with AHF and underlying renal dysfunction, neither low-dose dopamine nor low-dose nesiritide when added to diuretics enhanced decongestion or improved renal function. Further investigations of these, or other, AHF therapies should assess the potential for differential responses in HF with preserved versus reduced EF, stated Dr. Chen.
- © 2013 MD Conference Express®