Summary
It has been 15 years since the results of the United Kingdom Prospective Diabetes Study (UKPDS) were reported at the Barcelona European Association for the Study of Diabetes (EASD) 1998 Annual Meeting. This article discusses the global impact of the UKPDS findings, which have been reported in 82 publications.
- Diabetes Mellitus
- Diabetes Mellitus
- Endocrinology
- Diabetes & Metabolic Syndrome
- Exclusive Article - For home page
It has been 15 years since the results of the United Kingdom Prospective Diabetes Study (UKPDS) were reported at the Barcelona European Association for the Study of Diabetes (EASD) meeting. David Matthews, MA, DPhil, Oxford Centre for Diabetes, Endocrinology and Metabolism, Oxford, United Kingdom, discussed the global impact of the UKPDS findings, which have been reported in 82 publications.
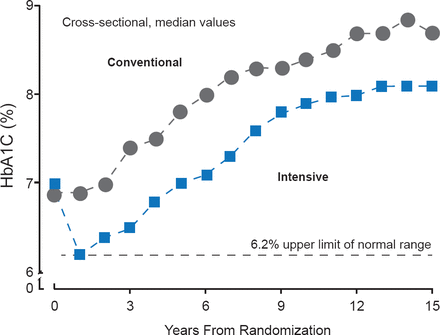
UKPDS, which was conducted in newly diagnosed patients with type 2 diabetes mellitus (T2DM) on monotherapy (for as long as possible), provided the first definitive evidence that intensive glycemic control could reduce microvascular events by a large (25%) and highly significant margin [UKPDS Study Group. Lancet 1998 (UKPDS 33)]. In this study, a policy of intensive glucose control maintained a median HbA1C of 7% over a median 10 years from diagnosis (Figure 1).
Effect of Conventional Versus Intensive Glucose Control on HbA1C
Reproduced from the UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352(9131):83853. With permission from Elsevier.
Over the 10 years of the study, HbA1C was 7.0% (range, 6.2% to 8.2%) in the intensive group compared with 7.9% (6.9% to 8.8%) in the conventionally treated group—an 11% reduction. This resulted in a 12% significant reduction in the risk of any diabetes endpoint (95% CI, 1% to 21%; RR, 0.88; p=0.029) and a 25% reduction in microvascular endpoints (95% CI, 7% to 40%; RR, 0.75; p=0.0099), as well as reductions in myocardial infarction (MI), cataract extraction, retinopathy, and albuminuria [UKPDS Study Group. Lancet 1998 (UKPDS 33)]. Importantly, the results of UKPDS have led to many additional studies in very different populations, the results of which continue to influence diabetes care. Current recommendations are that an HbA1C target of <7.0% is generally recommended, although a target of <6.5% may be reasonable for patients with a short duration of T2DM and without extensive atherosclerosis [Laakso M, Cederberg H. J Intern Med 2012].
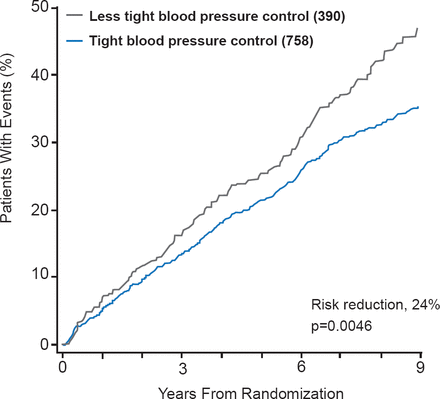
Elevated blood pressure (BP) may also increase the risk of diabetes-related events, and although tight BP control (130/80 mm Hg in patients with diabetes) has been shown to reduce the risk of diabetes events by 24% (p=0.0046; Figure 2) [UKPDS Study Group. BMJ 1998], the forthcoming Joint National Committee (JNC) 2013 Update is likely to recommend a hypertension treatment target of 140/90 mm Hg for all but older adults. Prof. Matthews noted that this is not that much different from the mean systolic BP in the UKPDS which was 144 mm Hg.
Effects of Tight Blood Pressure Control on Diabetes-Related Events
Adapted from UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). BMJ 1998; 317:703–713.
Epidemiology data support the concept that low HbA1C (<6%) and low systolic BP (<130 mm Hg) results in fewer diabetes-related endpoints [Adler AI et al. BMJ 2000; Stratton IM et al. BMJ 2000].
The UKPDS study addressed the issue of whether intensive glucose lowering with sulfonylureas increases the risk of cardiovascular mortality in patients with T2DM. Sulfonylurea (chlorpropamide, glibenclamide, or glipizide) or insulin substantially decreased the risk of microvascular complications, but not macrovascular disease compared with conventional treatment. Patients in the intensive treatment group had more hypoglycemic episodes (p<0.0001) and weight gain (p<0.001) [UKPDS Study Group. Lancet 1998 (UKPDS 33)]. Metformin was not associated with any weight increase compared with the conventionally treated group. Because of UKPDS the principle of intensive therapy was widely accepted and the concept of agent failure was switched to β-cell failure. The study also changed the thinking about β-blockers and the treatment of T2DM in patients with hypertension.
Another UKPDS study compared diabetes-related event outcomes in patients (some overweight) receiving sulfonylurea or metformin. Patients given metformin had improved diabetes-related endpoints (p=0.0034), all-cause mortality (p=0.021), and stroke (p=0.032) compared with patients receiving sulfonylurea or insulin. Metformin was also associated with less weight gain and fewer hypoglycemic events [UKPDS Study Group. Lancet 1998 (UKPDS 34)].
Virtually all guidelines quote the UKPDS as justification for using metformin as first-line therapy and the background medication on which all other pharmacotherapy are added citing its substantial effects on outcomes in overweight patients, the elderly, and its cost effectiveness. With the worldwide population of people with diabetes expected to grow to 330 million by 2030, data indicates morbidity can be reduced, lives prolonged, and blindness and renal failure prevented. Because of UKPDS, millions of people will have better outcomes and better lives.
In a continuation of this subject, Rury Holman, FMedSci, University of Oxford, Churchill Hospital, Headington, United Kingdom, presented the post-trial monitoring results of the UKPDS sulfonylurea plus metformin substudy.
The UKPDS provided the first definitive evidence that T2DM is a progressive condition, with increasing hyperglycaemia over time (Figure 1). As a result, a UKPDS substudy was introduced in which patients who continued to have fasting hyperglycemia despite maximal sulfonylurea therapy were randomized to remain on therapy with sulfonylurea alone or to have metformin added.
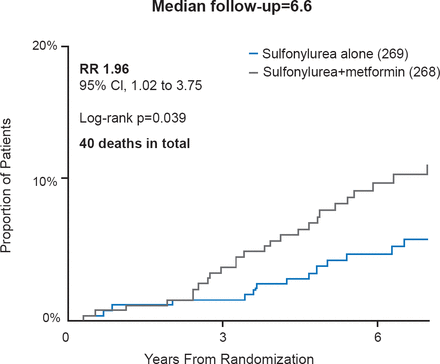
Given the main UKPDS findings demonstrating the beneficial effects of metformin on myocardial infarction and all-cause mortality it was a concern to the diabetes community when the sulfonylurea plus metformin substudy appeared to show a 96% increased risk of diabetes-related death (RR, 1.96; 95% CI, 1.02 to 2.75; p=0.039) compared with continued sulfonylurea alone (Figure 3) [UKPDS Lancet 1998 (UKPDS 34)]. The authors pointed out that the actual number of deaths were small; 26 in the sulfonylurea plus metformin group and 14 in the sulfonylurea-alone group. There were no differences in the incidence of MI, stroke, or microvascular events. The incidence of fatal and nonfatal events did not differ between the groups. Similar follow-up studies have produced conflicting results. Thus, a meta-analysis of nine studies that examined the association between combination therapy of sulfonylureas and metformin on risk of cardiovascular disease (CVD) or all-cause mortality between was conducted [Rao AD et al. Diabetes Care 2008].
Increase in Diabetes-Related Deaths
Reproduced from Rao AD et al. Is the Combination of Sulfonylureas and Metformin Associated With an Increased Risk of Cardiovascular Disease or All-Cause Mortality?: A meta-analysis of observational studies. Diabetes Care 2008;31:1672-1678. With permission from the American Diabetes Association.
In this study, the pooled RRs (95% CIs) of outcomes for individuals with T2DM prescribed combination therapy with sulfonylureas and metformin were 1.19 (0.88 to 1.62) for all-cause mortality, 1.29 (0.73 to 2.27) for CVD mortality, and 1.43 (1.10 to 1.85) for a composite endpoint of CVD hospitalizations or mortality (fatal or nonfatal events) [Rao AD et al. Diabetes Care 2008]. No significant effects with this combination therapy on either CVD mortality or all-cause mortality alone were evident.
New data presented at the EASD showed that during the 10-year post-trial monitoring of all surviving UKPDS patients the differential impact on diabetes-related death diminished and became statistically insignificant, while the hazard ratios for other prespecified clinical outcomes remained essentially unchanged.
- © 2013 MD Conference Express®