Summary
Many studies have shown that improved glucose control in patients with diabetes reduces microvascular complications. Dipeptidyl peptidase-4 (DPP-4) inhibitors improve glucose control and were thought to reduce the risk of cardiovascular events and all-cause mortality in patients with type 2 diabetes [Monami M et al. Diabetes Obes Metab 2013]. This article discusses the Examination of Cardiovascular Outcomes With Alogliptin Versus Standard of Care trial [EXAMINE; NCT00968708] and Saxagliptin Assessment of Vascular Outcomes Recorded in patients with diabetes mellitus [SAVOR]-TIMI 53 trial [NCT01107886] trials.
- Diabetes Mellitus
- Coronary Artery Disease
- Diabetes Mellitus
- Coronary Artery Disease
- Endocrinology
- Diabetes & Metabolic Syndrome
Many studies have shown that improved glucose control in patients with diabetes reduces microvascular complications. Dipeptidyl peptidase-4 (DPP-4) inhibitors improve glucose control and were thought to reduce the risk of cardiovascular (CV) events and all-cause mortality in patients with type 2 diabetes (T2DM) [Monami M et al. Diabetes Obes Metab 2013].
Two presentations from the Examination of Cardiovascular Outcomes With Alogliptin Versus Standard of Care trial [EXAMINE; NCT00968708] reported on the benefits of alogliptin versus placebo as add-on therapy to standard care for patients with T2DM and acute myocardial infarction or unstable angina (UA).
William B. White, MD, University of Connecticut School of Medicine, Farmington, Connecticut, USA, noted that after a median duration of follow-up of 18 months there were no significant differences between alogliptin and placebo in the primary endpoint of CV death, nonfatal myocardial infarction (MI), and nonfatal stroke.
This was a randomized double-blind, placebo-controlled, noninferiority study to demonstrate that major CV event rates were not higher with alogliptin compared with placebo in T2DM patients with recent acute coronary syndrome who are receiving standard of care for diabetes and secondary CV prevention. Secondary endpoints included an evaluation of the time from randomization to the first occurrence of the primary endpoint, urgent revascularization due to UA, and heart failure hospitalization.
Approximately 5400 men and women receiving antihyperglycemic therapy (mono- or combination therapy) were followed for up to 3.5 years post randomization. At baseline, median duration of diabetes was 7.1 years, median body mass index (BMI) was 29.7 kg/m2, mean HbA1C level was 8.0%±1.1% (range, 6.55%–11.0%). An MI was the qualifying event for study entry in most (77%) of patients.
The incidence of the primary endpoints between the two groups was not significantly different (placebo 11.8% vs 11.3% for alogliptin; HR, 0.96; one-sided repeated CI bound, 1.16).
There were no significant differences in secondary endpoints, the subgroup analysis, or rates of withdrawal or adverse events between the groups. Alogliptin did not increase major adverse CV event rates compared with placebo.
Simon Heller, MD, University of Sheffield, Sheffield, United Kingdom, reported alogliptin in patients with T2DM and recent acute CV problems was well tolerated and improved glycemic control without increasing rates of serious or nonserious hypoglycemia.
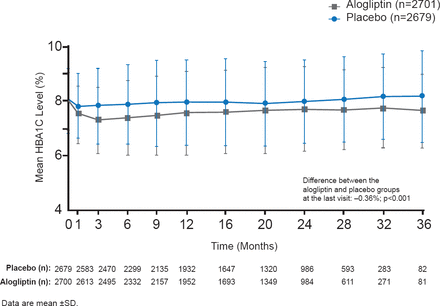
Patients were administered 25, 12.5, or 6.25 mg alogliptin (depending on kidney function) or placebo QD after randomization. HbA1C, fasting plasma glucose (FPG) levels, concomitant medications, body weight, adverse events, and lipids were measured at each study visit. Throughout the study, HbA1C was consistently lower and by the end of the study mean HbA1C was −0.36% lower compared with the placebo group (p<0.001; Figure 1).
Comparison of HbA1C Levels Over 36 Months in EXAMINE
Reproduced with permission from S Heller, MD.
Mean decreases from baseline FPG were significantly greater for alogliptin patients (−0.33 mmol/L; p<0.001). Significantly (p<0.001) more alogliptin patients achieved HbA1C levels <7.0% and <8.0% at 6, 12, 24 months, and last visit compared with placebo. Body weight remained stable in both groups. Serious hypoglycemia and pancreatitis were uncommon with no differences noted between the groups. Adverse events and initiation of dialysis were similar. There were no cases of pancreatic cancer.
Reports from the Saxagliptin Assessment of Vascular Outcomes Recorded in patients with diabetes mellitus [SAVOR]-TIMI 53 trial [NCT01107886] were presented by Itamar Raz, MD, Hadassah University Hospital, Israel and Deepak L Bhatt, MD, MPH, Brigham and Women's Hospital, Boston, Massachusetts, USA.
In the SAVOR trial, the DPP-4 inhibitor saxagliptin neither reduced nor increased the risk of the primary composite endpoint of CV death, nonfatal MI, or ischemic stroke compared with placebo. Patients (n=16,492) with T2DM and established CV disease (CVD) or multiple CV risk factors were randomized 1:1 to 5 mg saxagliptin or placebo once daily, then followed for a median duration of 2.1 years. Major secondary endpoints included the components of the composite endpoint, hospitalization for heart failure, UA, or coronary revascularization.
Enrolled patients were aged ≥40 years, had documented HbA1C ≥6.5% in the previous 6 months, and were at high risk for CV events. Baseline characteristics were similar between the two groups. Median duration of diabetes was 10 years and mean HbA1C was 8.0%. The majority of patients were also being treated with aspirin, statins, or ACE inhibitors for CV risk, and metformin for diabetes.
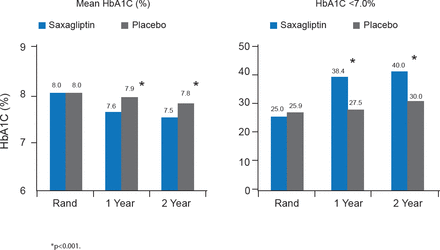
Mean HbA1C was significantly lower in patients treated with saxagliptin compared with placebo at 1 and 2 years, and significantly more saxagliptin-treated patients achieved HbA1C <7.0% at 1 and 2 years. These changes in glycemic control were in the context of a 23% decrease in the intensification of antihyperglycemic medications with saxagliptin compared with control (p<0.001), and a 30% decrease in the initiation of insulin therapy for >3 months with saxagliptin compared with control (p<0.001; Figure 2).
Glycemic Control at 1 and 2 Years in SAVOR
Rand=randomization.
Reproduced with permission from DL Bhatt, MD.
A primary endpoint event occurred in 613 patients in the saxagliptin group and in 609 patients in the placebo group (7.3% and 7.2%, respectively; HR, with saxagliptin, 1.00; 95% CI, 0.89 to 1.12; p=0.99 for superiority; p<0.001 for noninferiority.
Similar results were reported for the secondary endpoints. There were no significant differences for any of the individual endpoints except hospitalization for heart failure (2.8% with placebo vs 3.5% with saxagliptin; p=0.007). Subgroup analysis showed no differences. Rates of adjudicated cases of acute and chronic pancreatitis were similar in the two groups [Scirica BM et al. N Engl J Med 2013].
The investigators concluded that when added to standard of care for patients with T2DM and high CV risk saxagliptin improves glycemic control and after a median 2.1 years is noninferior to placebo for CV outcomes. They cautioned that no conclusions regarding longer treatment periods could be made. This study also was not designed to assess the impact of therapy on microvascular events.
In the second SAVOR presentation, the general safety of incretin-based therapy was proven, particularly the use of saxagliptin in patients with long disease duration, broad HbA1C levels, various antidiabetic medications, concomitant CVD, and other comorbidities. Saxagliptin improved glycemic control, prevented the progression of microalbuminuria, and decreased the need for the initiation of insulin therapy without an increased risk of hypoglycemia and major hypoglycemia, except in patients treated with sulfonylurea and with baseline HbA1C <7.0%. There were no increases in the rate of primary or secondary CV endpoints for patients treated with sulfonylurea or for hospitalization for hypoglycemia.
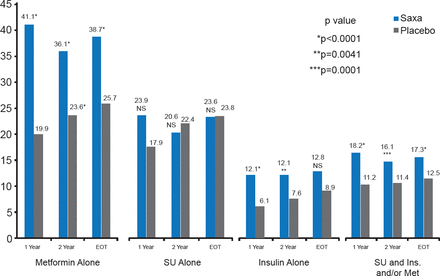
Significantly more patients treated with saxagliptin achieved HbA1C <7% without hypoglycemic events (excluding patients with HbA1C <7% at baseline) at 1 and 2 years and by end of trial regardless of ongoing treatment (Figure 3).
Proportion of Patients Achieving HbA1C <7% by Treatment
Ins=insulin; Met=metformin; Saxa=saxagliptin; SU=sulfonylurea.
Reproduced with permission from DL Bhatt, MD.
- © 2013 MD Conference Express®