Summary
White adipose tissue is thought to be critical in controlling the amount of fat in lean tissue because it takes up excess dietary fat to prevent potentially toxic dietary fatty acids from being metabolized by lean tissue. At least 4 different mechanisms are thought to contribute to the deposition of ectopic fat: increased dietary fat, increase uptake of nonesterified fatty acids from white adipose tissue lipolysis, impaired fatty acid oxidation, and increased lipogenesis. This article reviews research to understand abnormal organ-specific postprandial storage of dietary fatty acids, and the impact of lifestyle intervention to reduce this storage.
- Obesity
- Cardiometabolic Disorder
- Obesity
- Cardiometabolic Disorder
- Endocrinology
- Diabetes & Metabolic Syndrome
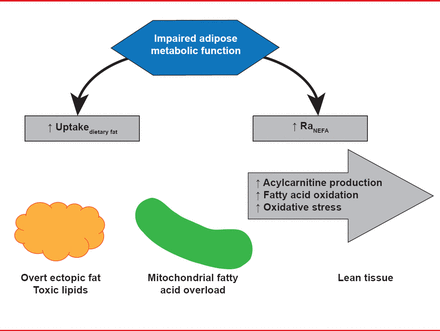
White adipose tissue is thought to be critical in controlling the amount of fat in lean tissue because it takes up excess dietary fat to prevent potentially toxic dietary fatty acids from being metabolized by lean tissue. Dietary fatty acid spillover drives, at least in part, regulation of ectopic fat, which plays a role in insulin resistance and metabolic abnormalities related to the metabolic syndrome. At least 4 different mechanisms are thought to contribute to the deposition of ectopic fat: increased dietary fat, increase uptake of nonesterified fatty acids from white adipose tissue lipolysis, impaired fatty acid oxidation, and increased lipogenesis (Figure 1).
Mechanisms for Ectopic Fat Deposition
Reproduced with permission from AC Carpentier, MD.
André C. Carpentier, MD, Université de Sherbrooke, Sherbrooke, Québec, Canada, reviewed research to understand abnormal organ-specific postprandial storage of dietary fatty acids, and the impact of lifestyle intervention to reduce this storage.
Using a novel approach with whole body positron emission tomography (PET), computed tomography (CT), and a fatty acid tracer (18F fluoro-6-thia-heptadecanoic acid [FTHA]), Prof. Carpentier and colleagues showed most dietary fatty acid uptake occurred 4 to 6 hours after eating. Dietary fatty acids first appear in the thoracic duct, and the greatest uptake per tissue mass was found (in order) in the liver, heart, kidneys, visceral adipose tissue, white adipose tissue, and resting skeletal muscles [Labbe SM. Am J Physiol Endocrinol Metab 2011].
Of the 9 subjects included in this study, those with impaired glucose tolerance (IGT), compared with those without IGT, were older (p<0.001), had higher body mass index (BMI; p<0.02) and waist circumference (p=0.008), more insulin resistance (p<0.001), fatty liver (p=0.10), and high triglycerides (TG; not significant). Postprandial metabolism was abnormal in patients with IGT Persons with IGT had significantly less dietary fatty acid uptake in the anterior abdominal subcutaneous tissue (p=0.05). Predictors of less dietary fatty acid uptake were waist circumference, fasting TG, postprandial glucose area under the curve (AUC), and nonesterified fatty acid AUC (likely related to nonesterified fatty acid spillover associated with impaired white adipose tissue and dietary fatty acid uptake). In subjects with prediabetes, dietary fatty acid uptake was reduced in visceral and white adipose tissue. Dietary fatty acid spillover was clearly associated with impaired dietary fatty acid uptake by visceral adipose tissue.
A striking and consistent finding was a significant increase in dietary fatty acids. Uptake of dietary fatty acid by the myocardium appeared to be maintained over time in persons with IGT However, there was no difference in liver uptake of dietary fatty acids in persons with and without IGT Thus, Prof. Carpentier concluded, it does not appear that dietary fat is distributed to a greater degree in the liver in diabetes and does not seem to contribute to its ectopic fat deposition. The best predictors of increased dietary fatty acid uptake by the myocardium were IGT and insulin resistance, whereas there was an inverse relation between fasting TG and liver dietary fatty acid uptake.
To determine the effect of weight loss on organ-specific dietary fatty acid storage, persons with IGT in the imaging study were enrolled in a 1-year lifestyle intervention. Modest reductions were achieved in weight (−3.7 kg), BMI (−1.1 kg/m2), and waist circumference (−5.0 cm). These reductions were associated with reductions in insulin resistance and postprandial insulin excursion, but IGT was not altered.
Subsequent imaging with FTHA showed that nonesterified fatty acids were significantly altered and TG were slightly reduced. Notably, dietary fatty acid uptake was decreased in the myocardium and increased in visceral adipose after weight loss. Prof. Carpentier hypothesized that weight loss is associated with at least partial normalization of impaired fat partitioning seen in IGT In contrast, a 7-day hypocaloric diet (−500 kcal, saturated fats <7% of total calories) increased dietary fatty acid partitioning to the myocardium, but did not alter partitioning to the liver, white adipose tissue, or visceral adipose tissue.
Although there is clear evidence that impaired dietary fatty acid storage in adipose tissue is associated with risk of developing cardiometabolic disorders (high TG and increased waist circumference), it is less clear whether this association is mechanistically related to impaired dietary fatty acid storage in other organs. Thus, the local factors regulating cardiac metabolism of dietary fat require further investigation.
- © 2013 MD Conference Express®