Summary
Obesity is an inflammatory process, and as visceral adipose tissue (VAT) increases, inflammation increases. As the components of metabolic syndrome increase, inflammation increases, as shown by increases in C-reactive protein (CRP) in the Women's Health Study [Ridker PM et al. Circulation 2003]. Additionally, this article discusses the role of epicardial and intrathoracic fat with cardiovascular (CV) risk factors, and the association between low levels of high-density lipoprotein cholesterol (HDL-C; <60 mg/dL) and increased hazard of coronary heart disease, stroke, and CV events.
- Prevention & Screening
- Cardiometabolic Disorder
- Obesity
- Lipid Disorders
- Diabetes Mellitus
- Prevention & Screening
- Cardiometabolic Disorder
- Endocrinology
- Diabetes & Metabolic Syndrome
- Obesity
- Lipid Disorders
- Diabetes Mellitus
Obesity is an inflammatory process, and as visceral adipose tissue (VAT) increases, inflammation increases, stated Peter Libby, MD, Brigham and Women's Hospital, Boston, Massachusetts, USA. As the components of metabolic syndrome increase, inflammation increases, as shown by increases in C-reactive protein (CRP) in the Women's Health Study [Ridker PM et al. Circulation 2003]. Evidence from the prospective observational Women's Health Study showed that inflammation precedes diabetes, with women in higher quartiles of CRP having a higher (nearly 4-fold) adjusted risk for incident diabetes [Pradhan AD et al. JAMA 2001].
Adipose tissue is teaming with metabolic activity and inflammatory cytokines, an “alphabet soup of mediators,” said Dr. Libby, most of which are proinflammatory. However, the anti-inflammatory adiponectin, which has protective effects on thrombosis, [Okamoto Y et al. Atherosclerosis 2013] is underproduced by VAT.
PET imaging has shown that glucose uptake is higher from VAT than from subcutaneous adipose tissue in lean and obese persons [Christen T et al. JACC Cardiovasc Imaging 2010]. A hint of important metabolic differences between VAT and subcutaneous adipose tissue which supported the imaging findings came from a mouse model in this study that showed hexokinase-1 activity was higher in VAT-derived, compared with subcutaneous adipose tissue-derived, stromal vascular cells during glucose uptake.
Innate immunity and specific cell populations in adaptive immunity may be important in visceral, versus lean, adipose tissue, and may be regulators of some inflammatory responses in VAT. TH1 cells produce interferon gamma (IFN-γ), which regulates inflammatory activation in VAT. IFN-γ is increased in high-fat, versus low-fat, fed mice [Rocha V et al. Cire Res 2008]. Obese adipose tissue contains regulatory T cells that affect metabolic parameters [Feuerer M. Nat Med 2009], and T-lymphocyte infiltration into VAT has been shown, which may contribute to local inflammatory cell activation [Kintscher U. Arterioscler Thromb Vase Biol 2008]. CD8+ effector T cells are activated in obese adipose tissue, which promotes recruitment and activation of macrophages into the tissue [Nishimura S et al. Nat Med 2009].
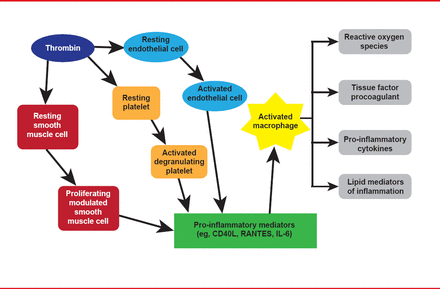
Obesity drives inflammation that underlies atherosclerosis initiation, progression, and complications, and diabetes. The vicious cycle between inflammation and thrombosis is illustrated in Figure 1. Plasminogen activator inhibitor 1 (PAI-1), the most important endogenous inhibitor of the fibrinolytic system, is a direct link between inflammation and thrombosis. Furthermore, PAI-1 is increased in diabetes and adipose tissue is thought to be one source of PAI-1.
Inflammation and Thrombosis Drive the Other in a Ongoing Cycle
Adapted from Croce K, Libby P. Curr Opin Hem 2007.
ROLE OF EPICARDIAL AND INTRATHORACIC FAT
In the setting of obesity, epicardial adiposity and VAT mass increase in concert, and an increase in either has a significant independent association with established cardiovascular (CV) risk factors, according to work reviewed by Amalia Gastaldelli, PhD, Institute of Clinical Physiology, CNR, Pisa, Italy, and University of Texas Health Science Center, San Antonio, Texas, USA.
On imaging, epicardial adiposity can be visualized as perivascular adipose tissue, surrounding coronary vessels, and, as with adipose tissue in other depots, it releases fatty acids and cytokines. Fat deposition also occurs in the intrathoracic region and inside cardiomyocytes. All of the cardiac fat depots have been shown to be markers of cardiac lipotoxicity, mitochondrial dysfunction, inflammation, local and systemic insulin resistance, atherosclerosis, and cardiac dysfunction, she stated.
Although cardiac fat is associated with impairment in heart metabolism and cardiac dysfunction, Prof. Gastaldelli stated that the interplay between cardiac fat accumulation, insulin resistance, and cardiac dysfunction remains to be fully established. Men accumulated more fat around their heart, with more intrathoracic and epicardial fat deposition, than women in a CT study [Rosito GA et al. Circulation 2008]. A study of men with untreated hypertension showed that increased levels of epicardial and visceral adipose tissue were independent from subcutaneous fat accumulation [Sironi AM et al. Hypertension 2004].
In the Framingham Heart Study women with higher levels of both epicardial adiposity and visceral fat had a higher prevalence of impaired fasting glucose and hypertension while in men the association was with metabolic syndrome [Rosito GA et al. Circulation 2008]. In general visceral fat was a stronger predictor than epicardial adiposity of cardiometabolic risk factors, probably because of its size.
An association was shown between cardiac fat accumulation and fatty liver, and between increased epicardial adiposity and reduced myocardial energy [Perseghin G. Hepatology 2008]. Epicardial adiposity thickness was correlated with endothelial dysfunction, as measured by reduced flow mediated vasodilation in patients with metabolic syndrome [Aydin H et al. Metab Syndr Relat Disord 2010]. Pericardial fat was an independent risk factor for coronary artery stenosis in the Korean Atherosclerosis Study 2 although the odd ratio was small when adjusted for age, gender, and body mass index (BMI; OR, 1.01; p<0.03) [Kim TH et al. Obesity (Silver Spring) 2011]. Table 1 details the relation between fat depots and CV disease burden in the Framingham Heart Study [Mahabadi AA et al. Eur Heart J 2009].
CVD Burden in Relation to Fat Deposition in the FHS
Epicardial adiposity increases in parallel to intrathoracic fat and BMI [Rosito G et al. Circulation 2008; Sironi et al. Diabet Med 2011; Yerramasu A et al. Atherosclerosis 2012]. Both epicardial adiposity and intrathoracic fat were shown to be associated with glucose metabolism and type 2 diabetes, decreased left ventricular diastolic function [Sironi AM et al. Hypertension 2008], and increased blood pressure in men with newly detected, untreated essential hypertension [Sironi AM et al. Hypertension 2004] and in the Framingham Heart Study [Rosito G et al. Circulation 2008].
RAISING HDL-C TO REDUCE CV RISK
Epidemiologic data have shown a robust association between low levels of high-density lipoprotein cholesterol (HDL-C; <60 mg/dL) and increased hazard of coronary heart disease, stroke, and CV events, according to Philip Barter, MD, PhD, University of New South Wales, Sydney, Australia. Low HDL-C (<42 mg/dL) remains associated with CV events even in patients with low-density lipoprotein cholesterol (LDL-C) levels, as shown by the TNT study [Barter P et al. N Engl J Med 2007]. Furthermore, HDL-C has several properties with the potential to inhibit development of atherosclerosis.
However, to date, interventions that raise HDL-C have not shown CV benefits in statin-treated patients. The AIM-HIGH and HPS2-THRIVE trials tested the benefit of raising HDL-C with niacin and failed to show a benefit. Prof. Barter stated AIM-HIGH was not powered to detect the 8% between-group difference in CV event rate expected from population-studies [Boden WE et al. N Engl J Med 2011], and the negative result in HPS2-THRIVE, presented at ACC. 13 in March 2013, was consistent with what would be predicted from modest changes in the lipid profile observed in the trial. Further, he stated the absence of a positive result in HPS2-THRIVE does not refute the hypothesis of significant beneficial effects with greater reductions in LDL-C or greater increases in HDL-C.
Trials with cholesteryl ester transfer protein (CETP) inhibitors that have been reported to date have also not shown a benefit. Torcetrapib was associated with adverse off-target pharmacology unrelated to CETP that likely was responsible for the negative result of ILLUMINATE, said Prof. Barter [Barter PJ et al. N Engl J Med 2007]. The dal-OUTCOMES trial with dalcetrapib, which increases HDL-C by about 30% with minimal effects on LDL-C levels, was terminated early for futility [Schwartz GG et al. N Engl J Med 2012]. Prof. Barter stated CETP inhibition may not be effective in patients treated soon after an acute coronary syndrome (ACS), which is supported by the observation that HDL-C isolated from patients after an ACS has impaired function, and by the unexpected observation in dal-OUTCOMES that the level of HDL-C in the placebo group did not predict CV risk.
The future of CETP inhibitors depends on the results of ongoing, clinical outcome trials with more potent agents without off-target effects. REVEAL HPS-3 TIMI 55 trial is testing anacetrapib in 30,000 patients with stable coronary artery disease and the ACCELERATE trial is testing evacetrapib in 11,000 patients with high-risk vascular disease.
- © 2013 MD Conference Express®