Summary
The main objective in caring for type 2 diabetes mellitus patients is the prevention of microvascular and potentially macrovascular complications with improved glycemic control along with management of other risk factors [Konig M et al. Curr Diabetes Rev 2013]. Diabetes is associated with both kinds of complications, affecting numerous organs, including the heart, brain, and kidneys [Cade WT. Phys Ther 2008]. This article discusses new ways to diagnose diabetes and identify those at high risk using HbA1C, dysglycemia in acute coronary syndromes, and diabetes and heart failure.
- Prevention & Screening
- Heart Failure
- Myocardial Infarction
- Diabetes Mellitus
- Hyperglycemia/Hypoglycemia
- Heart Failure
- Myocardial Infarction
- Prevention & Screening
- Cardiology & Cardiovascular Medicine
- Diabetes Mellitus
- Hyperglycemia/Hypoglycemia
According to the International Diabetes Federation, the number of people with the disease is increasing in every country. In 2011, diabetes affected 366 million individuals worldwide, and that figure is expected to rise to 552 million by 2030. Over 180 million people with diabetes (50%) are undiagnosed [International Diabetes Federation. IDF Diabetes Atlas: Fifth Edition, 2011. www.idf.org/diabetesatlas/5e/the-global-burden].
The main objective in caring for type 2 diabetes mellitus (T2DM) patients is the prevention of microvascular and potentially macrovascular complications with improved glycemic control along with management of other risk factors [Konig M et al. Curr Diabetes Rev 2013]. Diabetes is associated with both kinds of complications, affecting numerous organs, including the heart, brain, and kidneys [Cade WT. Phys Ther 2008]. While improving glycemic control has been shown to reduce microvascular complications, the impact on macrovacular complications has been less clearly established.
Glycated hemoglobin (HbA1C) is emerging as an important tool for formally diagnosing diabetes in the United States [American Diabetes Association. Diabetes Care 2010]. Screening for diabetes previously performed with standard fasting blood glucose or glucose challenge testing may now be done with HbA1C [Preiss D et al. Diabet Med 2011]. Naveed Sattar, MD, University of Glasgow, Glasgow, Scotland, United Kingdom, discussed new ways to diagnose diabetes and identify those at high risk using HbA1C.
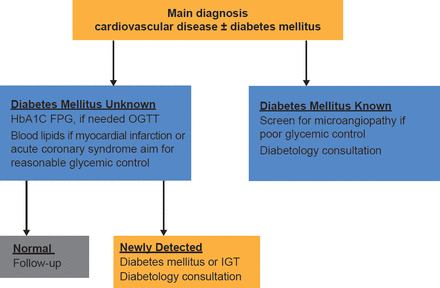
The adoption of HbA1C into diagnostic criteria will facilitate diabetic screening and may help refine assessment of cardiovascular risk (Figure 1) [Sattar N, Preiss D. Diabetologia 2012]. As a diabetes risk assessment and screening option, it requires no fasting and can dovetail with existing vascular screening. This makes it a very attractive way to greatly improve early detection and management of individuals at high risk of cardiovascular disease (CVD) and/or diabetes [Preiss D et al. Diabet Med 2011]. Based on such evidence, HbA1C has now been accepted into the European Society of Cardiology/European Association for the Study of Diabetes guidelines for the diagnosis of diabetes.
HbA1C as Diagnostic Criteria Will Facilitate Combined CV and Diabetic Screenings
CV=cardiovascular; FPG=fasting plasma glucose; IGT=impaired glucose tolerance; OGTT=oral glucose tolerance test.
Adapted from Sattar N, Preiss D. Diabetologia 2012.
As the interplay between CV risk and diabetes risk becomes clearer the case for combined diabetes/other CV risk factor screening (generally using HbA1C and nonfasting lipids) has now gained support [Sattar N. Diabetologia 2013]. Prof. Sattar discussed this and other new paradigms in play, including those that obviate conventional wisdom.
A meta-analysis challenged the belief that T2DM is a myocardial infarction risk equivalent [Sarwar N et al. Lancet 2010]. Rather, data showed that diabetes confers about a 2-fold excess risk for a wide range of vascular outcomes.
More recent data suggest that the duration of T2DM is associated with CVD; both early and late onset of diabetes are associated with increased risk; however, only early onset (associated with a duration >10 years) seems to portend risk equivalent to coronary heart disease [Wannamethee SG et al. Arch Intern Med 2011].
Whereas blood glucose was once considered a linear risk factor for CVD with a dose-response relationship [Levitan EB et al. Arch Intern Med 2004], Sarwar et al. [Lancet 2010] report that fasting blood glucose has a J-shaped relationship with vascular risk, with little if any risk at concentrations between 3.90 and 5.59 mmol/L, and risk escalating appreciably around current diagnostic cutoffs (ie, 7 mmol/L).
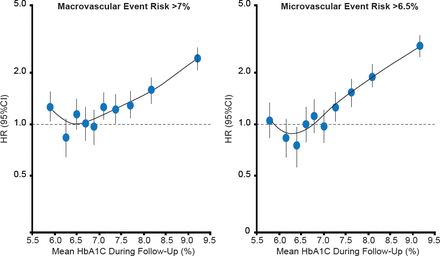
The ADVANCE trial investigated the relationship between HbA1C and the risk of vascular complications and death in patients with T2DM [Zoungas S et al. Diabetologia 2012]. The trial found that reducing HbA1C levels was associated with lower risks of microvascular events down to a threshold of 6.5%, and macrovascular events and death down to a threshold of 7.0% (Figure 2).
The Association of HbA1C With Risk in Diabetes
Adapted from Zoungas S et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia 2012;55(3):636–643.
UPDATE ON MANAGING DYSGLYCEMIA IN ACUTE CORONARY SYNDROMES
According to Anna Norhammer, MD, PhD, Karolinska University Hospital, Stockholm, Sweden, managing dysglycemia in the coronary care unit has evolved with insight from trials of glycemic control in the intensive care unit setting. The first DIGAMI trial, published in 1995, showed a clear mortality reduction with glucose control during and after the acute phase of a myocardial infarction, aiming at blood glucose levels between 7 to 10 mmol/L (plasma 7.7 to 11 mmol/L) during the acute phase. Since then trials from the intensive ward have reported conflicting results when it comes to lowering glucose during hospitalization [Malmberg K, Ryden L. J Am Coll Cardiol 1995].
In 2001, van den Berghe et al. [N Engl J Med] found that intensive insulin therapy to maintain blood glucose at or below 110 mg/dL reduced both morbidity and mortality among critically ill patients in the surgical intensive care unit (ICU). In 2009, Finfer et al. [N Engl J Med] demonstrated that intensive glucose control increased mortality among adults in the ICU, with a more lenient blood glucose target of ≤180 mg/dL resulting in lower mortality compared with a more intense target of 81 to 108 mg/dL. These data demonstrate a consistent pattern across care settings of increased CV risk associated with both hyperglycemia as well as hypoglycema.
This year's European Society of Cardiology Guidelines on Diabetes, Pre-diabetes, and Cardiovascular Diseases (developed in collaboration with the European Association for the Study of Diabetes) recommend that insulin-based glycemic control should be considered in acute coronary syndrome (ACS) patients with significant hyperglycemia of >180 mg/dL (>10 mmol/L), with the target adapted in the presence of comorbidities [Rydén L et al. Eur Heart J 2013]. In addition, glycemic control that may be accomplished by different glucose-lowering agents should be considered in DM and ACS.
Remaining questions, said Prof. Norhammer, include the role and optimal level of glycemic control for the outcome in ACS patients; and whether it is possible to reduce final infarct size by means of very early glucose-insulin-potassium administration after symptoms of myocardial infarction. Prof. Norhammar concluded that we should keep measuring glucose in the cardiac care unit and suggested that aiming at glucose levels between 7.8 to <11 mmol/L should be safe while the risk for hypoglycemia probably will increase if targeting values lower than 7 mmol/L. She also pointed out that a large proportion of patients will have previously unknown diabetes and impaired glucose intolerance, about 60% of myocardial infarction patients. This is only determined by performing an oral glucose tolerance test before discharge [Norhammer A et al. Lancet 2002].
DIABETES AND HEART FAILURE: WHAT ARE THE MANAGEMENT OPTIONS?
Some 5.7 million people in the United States have heart failure (HF) [Roger VL et al. Circulation 2012]. HF causes >55,000 deaths each year [Kochanek KD et al. Natl Vital Stat Rep 2011], with significant associated costs related to healthcare services, medications, and lost productivity [Heidenriech PA et al. Circulation 2011]. David Aguilar, MD, Baylor College of Medicine, Houston, Texas, USA, discussed the management options for DM and HF.
According to Dr. Aguilar, management of diabetes in patients with HF is generally is similar to those without HF, but there are diabetes-specific issues. These include the appropriate glycemic targets in patients with HF, and the appropriate options for hyperglycemic therapy.
Recommendations for glycemic control from the American Diabetes Association [Diabetes Care 2013] call for:
-
HbA1C goal below or around 7%, which is considered reasonable due to microvascular benefits and potential long-term macrovascular benefits if glucose lowering regimen implemented soon after DM diagnosis
-
More stringent HbA1C goals (<6.5%) for selected patients (eg, those with short diabetes duration, less risk of hypoglycemia, long life expectancy, and no significant CVD)
-
Less stringent HbA1C goals (<8%) for those with advanced microvascular or macrovascular complications, extensive comorbid conditions, or a history of hypoglycemia or difficult to treat, longstanding DM
According to Dr. Aguilar, optimal hyperglycemic therapy in HF patients is not clear. He noted that metformin can be used with caution, and that prospective outcome data are needed for other agents, including glucagon-like peptide-1 agonists and dipeptidyl peptidase-4 inhibitors given unexpected results from the SAVOR-TIMI 53 trial in high-risk CV patients (see article on page 17).
- © 2013 MD Conference Express®