Summary
Renal denervation (RDN) is a promising emerging treatment option for resistant hypertension, as well as other diseases that appear to be associated with sympathetic activation. This article discusses guideline recommendations for the diagnosis and conventional treatment of resistant hypertension; data of RDN efficacy and safety; investigational indications for RDN therapy; as well as the reasons behind the popularity and success of RDN.
- Interventional Radiology
- Hypertensive Disease
- Renal Disease
- Hypertension & Kidney Disease
- Interventional Radiology
- Hypertensive Disease
- Renal Disease
- Cardiology & Cardiovascular Medicine
- Hypertension & Kidney Disease
Renal denervation (RDN) is a promising emerging treatment option for resistant hypertension, as well as other diseases that appear to be associated with sympathetic activation. Alexandra O. Konradi, MD, PhD, Almazov Federal Center for Heart, Blood and Endocrinology, St. Petersburg, Russia, presented guideline recommendations for the diagnosis and conventional treatment of resistant hypertension.
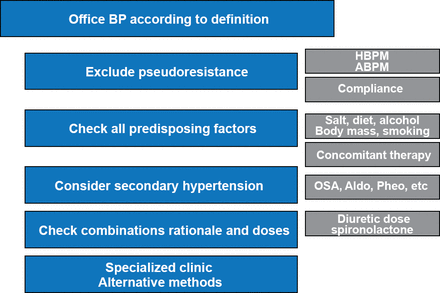
Prof. Konradi highlighted an algorithm adapted from the 2008 American Heart Association guidelines for resistant hypertension [Calhoun DA et al. Hypertension 2008] that addresses many important underlying issues in the diagnosis and management of resistant hypertension (Figure 1). According to the 2013 European Society of Hypertension/European Society of Cardiology guidelines, resistant hypertension can be caused by lifestyle factors such as obesity, excessive alcohol or sodium intake, chronic use of vasopressor or sodium-retaining agents, obstructive sleep apnea (OSA), secondary forms of hypertension, or advanced or irreversible organ damage [Mancia G et al. J Hypertens 2013]. Therefore, physicians should screen patients for OSA and agents that can increase blood pressure (BP), including nonsteroidal anti-inflammatory drugs, diet pills, and oral contraceptives among others. In addition, treatment adherence is highly important and should be assessed routinely [Mancia G et al. J Hypertens 2013]. In addition, physicians should exclude secondary causes of hypertension when appropriate and continually work to optimize antihypertensive regimens.
Algorithm to Validate Resistant Hypertension in Presenting Patients
ABPM=ambulatory blood pressure monitoring; Aldo=aldosteronism; BP=blood pressure; HBPM=home blood pressure measurement; OSA=obstructive sleep apnea; Pheo=pheochromocytoma.
Adapted from Calhoun DA et al. Hypertension 2008.
Markus Schlaich, MD, PhD, Neurovascular Hypertension & Kidney Disease Laboratory, Melbourne, Australia, discussed the principles underlying RDN as a treatment of resistant hypertension. When the sympathetic nervous system is overactive, norepinephrine acts to stimulate the kidneys, heart, veins, and arterioles. Sympathetic stimulation of the kidneys results in sodium retention, renin release, and vasoconstriction. Stimulation of the heart increases heart rate and stroke volume, with chronic activation by norepinephrine leading to arrhythmias and left ventricular hypertrophy. In addition, norepinephrine affects the metabolism by stimulating lipolysis in adipocytes, gluconeogenesis in the liver, altering insulin release by the pancreas and rarefaction of the skeletal arterioles.
Prof. Schlaich highlighted multiple factors that can cause chronic activation of the sympathetic nervous system. For example, obesity may be associated with chronic sympathetic activation and it has been observed that individuals who lose ∼8% to 9% of their body weight through diet or diet plus exercise demonstrate a significant decrease in sympathetic nerve activity [Straznicky NE et al. Diabetes 2010]. Moreover, ∼70% to 80% of patients with resistant hypertension also have OSA, and OSA is associated with high sympathetic nervous activation.
Henry Krum, MBBS, PhD, Centre of Cardiovascular Research & Education in Therapeutics, Monash University, Melbourne, Australia, presented data of RDN efficacy and safety. The Symplicity HTN Program included three trials that evaluated RDN in refractory hypertension. In Symplicity HTN-1, 153 patients with ≥160 mm Hg systolic BP (SBP) despite treatment with ≥3 antihypertensive agents and an estimated glomerular filtration rate (eGFR) of ≥45 mL/min/1.73 m2 received RDN [Symplicity HTN-1 Investigators. Hypertension 2011; Krum H et al. Lancet 2009]. Patients undergoing RDN had reductions in office BP with a mean change from baseline of −27 mm Hg in SBP and −17 mm Hg in diastolic BP (DBP) at 12 months (p<0.001) and −32 and −14 mm Hg in SBP and DBP, respectively, at 36 months (in those patients for which follow up was available 88 patients, 58%).
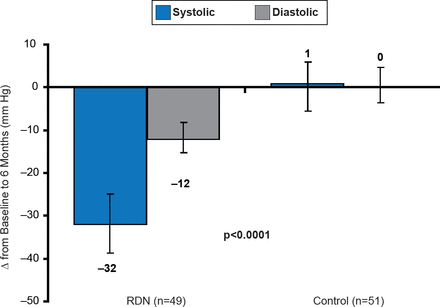
In the Symplicity HTN-2 Study, 106 patients with uncontrolled hypertension of ≥160 mm Hg SBP despite ≥3 antihypertensive agents were randomized to receive RDN or control treatment [Esler MD et al. Lancet 2010]. Patients that received RDN demonstrated a 32 and 12 mm Hg reduction in SBP and DBP, respectively, compared with baseline at 6 months (p<0.0001), compared with a +1 mm Hg in SBP and no change in DBP in patients who received the control treatment (Figure 2). The randomized Symplicity HTN-3 trial is currently ongoing with results expected in 2016.
Effect of RDN on Office BP in Symplicity HTN-2
RDN=renal denervation.
Reproduced from Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010;376(9756):1903–1909. With permission from Elsevier.
Similar results were demonstrated in the EnLigHTN trial, which used a different catheter for the RDN procedure. In the EnLigHTN study, RDN was associated with a 26 and 10 mm Hg decrease in office-based SBP and DBP, respectively, compared with baseline [Worthley SG et al. Eur Heart J 2013]. Prof. Krum pointed out that multiple trials support the benefits of RDN in resistant hypertension though acknowledged that there are several case reports demonstrating no effect of RDN or even a paradoxical increase in BP [Baumbach A et al. Int J Cardiol 2013; Persu A et al. J Hypertens 2013 (abstr LB01.06); Vonend O et al. Lancet 2012]. Still further, Prof. Krum noted that there needs to be more robust safety analyses and suggested that the findings of the Symplicity HTN-3 trial would be very important. There were 4 complications out of 153 patients in Symplicity HTN-1, which included renal artery dissection and access site complications [Symplicity HTN-1 Investigators. Hypertension 2011]. In addition, reports of renal artery stenosis, hypotensive episodes, hypertensive episodes, and death due to myocardial infarction, sudden cardiac death, or cardio-respiratory arrest have been published.
Importantly, ∼10% of the patients in Symplicity HTN-2 did not respond to RDN [Esler MD et al. Lancet 2010]. Prof. Mancia pointed out that nonresponse may be due to a variety of factors such as nonsympathetic factors or incomplete RDN. Therefore, it is important to determine which patients will benefit from RDN and the completeness of the denervation should be assessed.
Michael Böhm, MD, PhD, Saarland University Hospital, Homburg/Saar, Germany, presented other investigational indications for RDN therapy. These indications include atrial fibrillation (AF), ventricular arrhythmias, insulin resistance and diabetes, OSA, chronic kidney disease, and chronic heart failure.
In the Symplicity trials, it was observed that some patients that had received RDN experienced improved blood glucose control [Böhm M et al. EuroIntervention 2013]. One hypothesis states that due to vasoconstriction, blood flow is directed away from insulin-sensitive organs such as the skeletal muscle, which can lead to insulin resistance. RDN is thought to restore the blood flow to insulin-sensitive organs, thus improving insulin sensitivity. In a pilot study of Symplicity data, patients with impaired fasting glucose that received RDN demonstrated a significant improvement in fasting glucose, fasting insulin, and the Homeostasis Model of Assessment-Insulin Resistance index from baseline compared with control patients at 1 and 3 months [Mahfoud F et al. Circulation 2011].
In a study of patients with moderate to severe chronic kidney disease, RDN resulted in stabilization of the disease as measured by eGFR [Hering D et al. J Am Soc Nephrol 2013]. In chronic heart failure, renal norepinephrine spillover is associated with significantly greater cumulative mortality (p=0.003), whereas total body spillover was not significantly associated (p=0.2) [Petersson M et al. Eur Heart J 2005]. In AF, RDN appears to decrease left ventricular hypertrophy beginning at 1 month following the intervention [Brandt MC et al. J Am Coll Cardiol 2012]. RDN also appeared to improve OSA; in a porcine model, RDN led to a reduction in atrial effective refractory period-shortening and AF-inducibility [Linz D et al. Hypertension 2012]. In a porcine model of ventricular arrhythmia, RDN resulted in reduced extra beats due to acute ventricular ischemia [Linz D et al. Hearth Rhythm 2013]; however, reperfusion-induced arrhythmias are not affected by RDN.
Guiseppe Mancia, MD, PhD, University of Milano-Biccoca, Monza, Italy, discussed the reasons behind the popularity and success of RDN, which include a robust pathophysiological rationale and evidence in small studies of a durable decrease in office BP over 3 years. However, Prof. Mancia warned that the long-term efficacy and safety of RDN on BP control has not yet been demonstrated and important questions remain about fiber regeneration and the long-term safety of multiple renal artery interventions.
- © 2013 MD Conference Express®