Summary
Results from the Cozaar in Marfan Patients Reduces Aortic Enlargement trial [COMPARE; Groenink M et al. Eur Heart J 2013] demonstrated that the angiotensin receptor blocker, losartan, significantly reduced the rate of aortic enlargement after 3 years in patients with Marfan syndrome.
- Cardiology Clinical Trials
- Cardiology Genomics
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
- Cardiology Genomics
Results from the Cozaar in Marfan Patients Reduces Aortic Enlargement trial [COMPARE; Groenink M et al. Eur Heart J 2013] reported by Maarten Groenink, MD, PhD, Academic Medical Centre, Amsterdam, The Netherlands, demonstrated that the angiotensin receptor blocker, losartan, significantly reduced the rate of aortic enlargement after 3 years in patients with Marfan syndrome (MFS).
MFS is a connective tissue disorder caused by a mutation in fibrillin-1 that is associated with structural dysfunction in the aortic wall and biochemical changes including over expression of TGF β [Cohn RD et al. Nat Med 2007]. Patients with MFS are at an increased risk of sudden death due to aortic dissection or rupture. Clinical management includes prophylactic aortic root replacement and pharmacologic therapy (β-blockers and possibly losartan).
COMPARE was a multicenter, open-label, randomized, controlled trial designed to assess the effect of the addition of losartan to the standard of care on the rate of aortic dilatation at any level in adult patients with MFS. The study included adults aged ≥18 years with MFS (as classified by the 1996 Ghent criteria) with an aortic root diameter <50 mm, no aortic dissection and ≤1 vascular prosthesis. Subjects received losartan (100 mg QD) along with their previous medication (n=116) or remained on their previous medication only (n=117). Magnetic resonance imaging was performed at inclusion and after 3 years of follow-up. The primary endpoint was aortic dilatation rate at any of the predefined aortic levels at follow-up. Secondary endpoints included changes in the incidence of cardiovascular mortality, aortic dissection, aortic volume, and prophylactic aortic surgery [Radonic T et al. Trials 2010].
Subjects (53% women in the control group; 41% in the treatment group) were aged 20 to 50 years (mean age 37 years); most (∼73%) were on β-blockers. A significant proportion of patients in both groups had already undergone aortic root replacement (31% of controls and 23% in the treatment group). At baseline, subjects had a mean aortic root measurement of 44 to 45 mm.
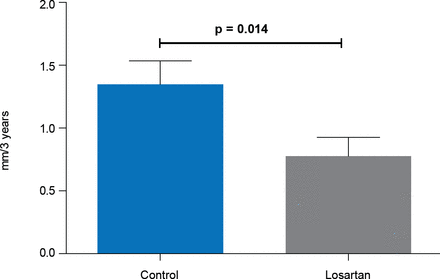
After 3 years aortic root enlargement was significantly less in the losartan group than in the control group (0.77 vs 1.35 mm; p=0.014; Figure 1), and 50% of losartan patients showed no growth of the aortic root compared with 31% of controls (p=0.022).
Aortic Root Dilatation Rate
Reproduced with permission from M Groenink, MD, PhD.
All subgroups benefited from losartan regardless of age, sex, the presence of fibrillin-1 mutation, β-blocker use, mean aortic pressure, or aortic root size. There were no differences in aortic dilatation rate beyond the aortic root. There were no significant differences in combined clinical endpoints between the two groups. There were no cardiovascular deaths in either arm.
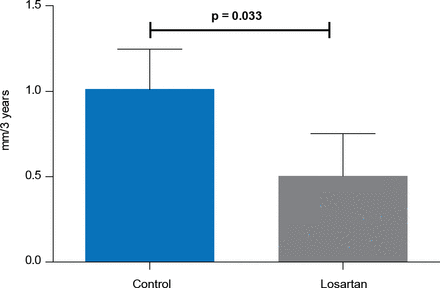
In a small subset of patients treated with prior aortic root replacement, patients treated with losartan (n=26) had significantly lower dilatation rates of the aortic arch compared with controls (n=31; 0.50 vs 1.01 mm; p=0.033; Figure 2).
Dilatation Rate of the Aortic Arch After Prophylactic Aortic Root Replacement
Reproduced with permission from M Groenink, MD, PhD.
The results of the COMPARE trial suggest that the addition of losartan to standard care in patients with MFS reduces the rate of aortic dilatation and may also reduce the rate of aortic arch dilatation among patients who have already had aortic root replacement. Study limitations include being open-label and not achieving target inclusion population.
- © 2013 MD Conference Express®