Summary
This article presented data from an international multicenter study to evaluate a cardiac valve designed for placement without the need for a median sternotomy. Transcatheter aortic valve replacement (TAVR) performed as well as surgical aortic valve replacement (SAVR), and improved survival at 1 and 2 years compared with medical management in patients with severe aortic stenosis (AS). The Placement of Aortic Transcatheter Valve trial [PARTNER; Leon MB et al. N Engl J Med 2010] was designed to investigate the safety and effectiveness of TAVR in patients with severe AS.
- Interventional Techniques & Devices
- Valvular Disease
- Interventional Techniques & Devices
- Valvular Disease
- Cardiology
Matthews Chacko, MD, Johns Hopkins Hospital, Baltimore, Maryland, USA, presented data from an international multicenter study to evaluate a cardiac valve designed for placement without the need for a median sternotomy. Transcatheter aortic valve replacement (TAVR) performed as well as surgical aortic valve replacement (SAVR), and improved survival at 1 and 2 years compared with medical management in patients with severe aortic stenosis (AS).
Classic symptoms of AS include angina, syncope, and heart failure (HF). Once these symptoms develop, the prognosis is extremely poor. Patients typically die within 5 years of angina onset, 3 years after the onset of syncope, and 2 years after the onset of HF.
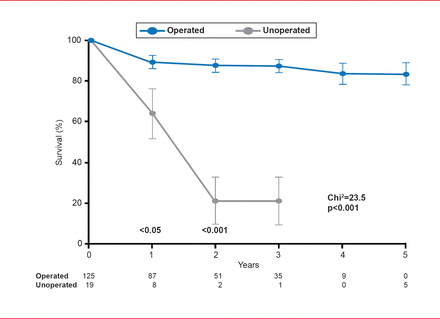
Surgical aortic valve replacement (SAVR) has led to marked long-term survival benefit in patients with symptomatic AS (Figure 1) [Schwarz F et al. Circulation 1982]. However, not all patients can tolerate surgery, and for individuals who are not candidates for SAVR, TAVR offers a less invasive treatment option [Leon MB et al. N Engl J Med 2010].
Survival Benefit of SAVR in Patients With Severe Aortic Stenosis
Reproduced from Schwarz F et al. The effect of aortic valve replacement on survival. Circulation 1982;66(5):1105–1110. With permission from Lippincott, Williams and Wilkins.
The Placement of Aortic Transcatheter Valve trial [PARTNER; Leon MB et al. N Engl J Med 2010] was designed to investigate the safety and effectiveness of TAVR in patients with severe AS. This technology involved a heart-valve system comprising a balloon-expandable, stented bioprosthesis designed to be delivered through the transfemoral route [Leon MB et al. N Engl J Med 2010].
PARTNER was a multicenter, prospective trial that was conducted among 25 centers in the United States, Canada, and Germany. Following screening of 3105 individuals with AS, 1058 patients were enrolled and randomized to two large cohorts that represented individually powered parallel studies. Of the 3105 individuals originally screened, 358 patients were randomized as part of the PARTNER trial and were assigned to a cohort of patients who were considered unsuitable candidates for SAVR (Cohort B). The remaining 700 individuals were assigned to a cohort of patients who, despite high surgical risk, were still considered candidates for SAVR (Cohort A) [Leon MB et al. N Engl J Med 2010].
The primary endpoints of the study were all-cause mortality over the duration of the trial, and the rate of the composite endpoint of death from any cause, or repeat hospitalization due to valve- or procedure-related clinical deterioration. Secondary endpoints included the rate of death from cardiovascular causes, NYHA functional class, valve performance (assessed by echocardiography), and the rate of problems such as major strokes and vascular complications [Leon MB et al. N Engl J Med 2010].
At 1 year, there was a significant difference in the rate of death from any cause between patients treated with TAVR compared with standard therapy, with a significantly lower incidence in the TAVR arm (30.7% vs 50.7%; HR, 0.55; 95% CI, 0.40 to 0.74; p<0.001). The rate of the composite endpoint of death from any cause or repeat hospitalization was also significantly lower in the TAVR arm than in the standard therapy arm (42.5% vs 71.6%; HR, 0.46; 95% CI, 0.35 to 0.59; p<0.001) [Leon MB et al. N Engl J Med 2010].
The rate of cardiac symptoms (NYHA functional class III or IV) was also lower among patients in the TAVR arm who had survived to 1 year, compared with those receiving standard therapy (25.2% vs 58.0%; p<0.001). However, at 30 days, the incidence of major strokes (5.0% vs 1.1%; p=0.06) and major vascular complications (16.2% vs 1.1%; p<0.001) were significantly increased in patients who underwent TAVR compared with standard medical therapy, the latter of which was particularly attributed to the large size of femoral access sheaths necessary to insert this system. Valve performance, as evaluated by echocardiography, had not deteriorated during the year following implantation in TAVR patients [Leon MB et al. N Engl J Med 2010].
Longer term data from this study, presented at the Transcatheter Cardiovascular Therapeutics annual meeting in 2012, also reinforced the 1-year results, demonstrating reduced mortality at 24 months post procedure in the TAVR patients compared with those receiving standard medical treatment (43.0% vs 68.0%), as well as decreased need for hospitalization. TAVR was also associated with a significantly increased incidence of major strokes in patients, compared with standard therapy (13.8% vs 5.5%), as well as improved NYHA functional class [Kapadia S. TCT 2012].
Additionally, 2-year outcomes for patients in Cohort A also demonstrated benefit in the use of TAVR in patients with severe AS. All-cause mortality in patients treated with TAVR was similar to that for SAVR (HR, 0.90; 95% CI, 0.71 to 1.15; p=0.41), and the rates of death were similar at 2 years (33.9% and 35.0%, respectively). The risk of stroke remained higher in patients randomized to TAVR compared with those in the SAVR arm at 2 years (7.7% vs 4.9%). NYHA functional class improvement was comparable in both TAVR and SAVR groups of surviving patients at 2 years (1.72 vs 1.70; p=0.87), and most patients had NYHA Class I or II status (83.9% vs 85.2%). Aortic regurgitation occurred more commonly following TAVR than SAVR (6.9% vs 0.9%; p<0.001), and was associated with increased late mortality (HR, 2.11; 95% CI, 1.43 to 3.10; p<0.001) [Kodali SK et al. N Engl J Med 2012].
Dr. Chacko concluded that although data have thus far demonstrated significant benefit of TAVR in high-risk patients, the technology still requires further study in expanded patient populations. He highlighted the importance of a team approach to TAVR and stressed that reducing the incidence of vascular access complications, paravalvular leaks and periprocedural strokes will be key in advancing this technology and improving outcomes in patients with severe AS.
- © 2013 MD Conference Express®