Summary
The first Caribbean studies to examine the performance of Abbott's experimental bioresorbable vascular scaffold indicate that the device is feasible to be used in clinical practice.
- Interventional Techniques & Devices Clinical Trials
- Interventional Techniques & Devices
- Cardiology & Cardiovascular Medicine
- Cardiology Clinical Trials
The first Caribbean studies to examine the performance of Abbott's experimental bioresorbable vascular scaffold (BVS) indicate that the device is feasible to be used in clinical practice, according to a presentation by Ingrid Valdez, MD, Los Centros de Diagnóstico y Medicina Avanzada y de Conferencias Médicas y Telemedicina (CEDIMAT), Santo Domingo, Dominican Republic.
As of July 2013, eight patients in the Dominican Republic, who are the first in the Caribbean to receive the device for use, have been treated with the bioresorbable stents. Dr. Valdez presented data from the first five patients for whom 30-day follow-up is available.
Bioresorbable stents are absorbed by the vessel wall over time and evidence of the coronary stent disappears. Thus, it is possible that the normal vascular functions of the vessel can be restored once the stent is absent. The results presented demonstrated the use of the experimental device in four men and one woman. The patients were aged between 39 and 75 years, and all had multivessel coronary artery disease. Dyslipidemia was the most frequent risk factor and was found in 80% of the patients. Hypertension was present in 60% of the patients, and 20% of the patients had diabetes. Ongoing tobacco abuse was present in 20%. Additional angiographic characteristics are outline in Table 1.
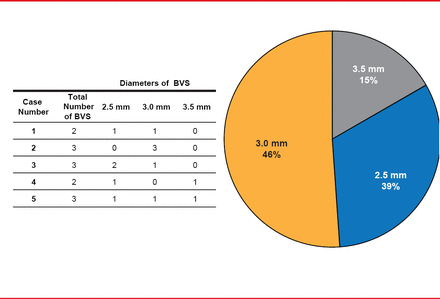
For most patients, it was necessary to use more than one device of varying diameters to cover the entire lesion (Figure 1). In one patient, there was one complication: a perforation caused by the guidewire during the predilation phase. In that case, Dr. Valdez noted, physicians performed a double coil implantation, and then successfully performed the remainder of the stenting procedure.
Angiographic Characteristics
Number of BVSs Used
BVS=bioresorbable vascular scaffold.
Reproduced with permission from I Valdez, MD.
Like the XIENCE V which is a drug-eluting stent using a traditional scaffold, the Abbott BVS serves as a vehicle for everolimus. The efficacy of everolimus with stenting for reducing restenosis has demonstrated in trials of the XIENCE V stent. The delivery platform of the Abbot BVS is the same as the XIENCE V. Although the biosorbable scaffold is not yet approved by the United States Food and Drug Administration, 12-month data from the Spirit I, II, and III trials in more than 2000 patients have demonstrated bioresorbable stents to be noninferior to the Xience V (MACCE at 12 months) [Serruys PW et al. Eurointervention 2005, 2006; Stone GW et al. JAMA 2008].
Initial experience with the Abbott BVS demonstrate that implantation is possible even in some complex cases. Thus, these results from the first-ever trials of the device in the Caribbean are consistent with prior studies that have found use of the bioresorable devices to be feasible. Further studies are needed to better understand the long-term clinical outcomes in patients treated with bioresorbable stents.
- © 2013 MD Conference Express®