Summary
This article discusses the relationship between chronic kidney disease (CKD) and cardiovascular disease (CVD), and provided an overview of the SHARP trial, the largest study of lipid-lowering therapy in patients with CKD and CVD.
- Lipid Disorders
- Renal Disease Clinical Trials
- Hypertension & Kidney Disease
- Lipid Disorders
- Cardiology & Cardiovascular Medicine
- Renal Disease
- Cardiology Clinical Trials
- Hypertension & Kidney Disease
Robert P. Giugliano, MD, SM, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA, discussed the relationship between chronic kidney disease (CKD) and cardiovascular disease (CVD), and provided an overview of the SHARP trial, the largest study of lipid-lowering therapy in patients with CKD and CVD.
The National Kidney Foundation (NKF) defines CKD as kidney damage for ≥3 months with structural or functional abnormalities of the kidney, manifested either on pathology, or by clinical markers of kidney damage (eg, elevated creatinine). The patient may have a reduced glomerular filtration rate (GFR) <60 mL/min/1.73 m2 for ≥3 months as well. In the United States, the vast majority of CKD patients are equally split between NKF Stages 1, 2, and 3 (GFR >90, 60 to 89, and 30 to 59 mL/min/1.73 m2, respectively), with only ∼0.3% falling into Stages 4 and 5 (GFR of 15 to 29 and <15 mL/min/1.73 m2, respectively).
Diabetes and hypertension are the traceable causes of CKD in about two thirds of cases in the United States [http://www.kidney.org/kidneydisease/aboutckd.cfm. Accessed August 22, 2013]. Renal disease itself raises CV risk, and the severity of CKD is associated with the severity of CV risk.
According to data from the Kaiser Permanente Renal Registry of 1,120,295 adults, kidney function showed a linear increase in the adjusted risk of any CV event as the estimated GFR (eGFR) decreased [Go AS et al. N Engl J Med 2004].
The link between CKD and CV death is even stronger, with exponential increases in risk.
A GFR of 60 is associated with a 2-fold risk of CV death, while a GFR of 30 carries a 4-fold increase in risk. There is a 10- to 30-fold increased risk in patients on dialysis.
AGGRESSIVE LIPID LOWERING IN CKD
The results of three different observational studies have suggested that patients with CKD should receive aggressive lipid-lowering therapy. The US Renal Data System Morbidity and Mortality Wave 2 study reported a 36% reduction in CV death (RR, 0.64; 95% CI, 0.45 to 0.91) in the 9.7% of patients on a statin [Seliger SL et al. Kidney Int 2002]. The prospective, observational Dialysis Outcomes and Practice Patterns Study from dialysis centers across seven countries showed a significant 23% reduction in cardiac mortality (p=0.03) in the 11.8% of patients taking a statin [Mason NA et al. Am J Kidney Dis 2005]. Finally, the Pravastatin Pooling Project, a meta-analysis of clinical trials with pravastatin versus placebo, found a reduction in the composite of coronary heart disease death, myocardial infarction (MI), or revascularization (HR, 0.77; 95% CI, 0.68 to 0.86) and a decrease in total mortality (HR, 0.86; 95% CI, 0.74 to 1.00; p=0.045) in 4991 patients with Stage 3 CKD [Tonelli M et al. Circulation 2004]. Although the results from each study were hypothesis-generating, Dr. Giugliano maintained that they did not provide definitive evidence.
However, two double-blind, placebo-controlled clinical trials of statin therapy in patients with CKD have been conducted. The 4D study of 1255 diabetic patients (aged 18 to 80 years) on dialysis <2 years showed a 42% reduction in low-denisity lipoprotein cholesterol (LDL-C) with atorvastatin 20 mg daily, but demonstrated no significant difference for the primary outcome of cardiac death, MI, or stroke at 4 years (RR, 0.92; 95% CI, 0.77 to 1.10; p=0.37) [Wanner C et al. N Engl J Med 2005]. The AURORA study also showed a reduction in LDL-C, by 43% with rosuvastatin 10 mg, but there was no significant reduction in the primary endpoint of CV death, MI, or stroke at 4 years (HR, 0.96; 95% CI, 0.84 to 1.11; p=0.59) [Fellström BC et al. N Engl J Med 2009]. The AURORA study patients, with or without diabetes, were older on average (aged 50 to 80 years) and had been on dialysis >3 months. There were no significant differences for any of the individual endpoints in either study.
THE SHARP TRIAL OF LIPID-LOWERING IN CKD
The Study of Heart and Renal Protection [SHARP] evaluated a broader population of CKD patients [SHARP Collaborative Group. Am Heart J 2010]. Only about one third of patients were on dialysis. Eligible patients were aged ≥40 years, had CKD, had an elevated creatinine on at least two occasions (if not receiving dialysis; men ≥1.7 mg/dL; women ≥1.5 mg/dL), and had no history of MI or coronary revascularization. Equipoise was required, as LDL-lowering treatment was not indicated, nor was it contraindicated.
Patients were randomized to a combination of ezetimibe 10 mg plus simvastatin 20 mg daily (eze/simv; n=4193) or placebo (n=4191). For 1 year, 1054 patients received simvastatin 20 mg daily alone to assess safety, and were then randomized to eze/simv. The final analysis included 4650 patients on eze/simv and 4620 patients on placebo at a median follow-up of 4.9 years.
The study population was typical for moderate to severe renal disease. They had a mean age of 62 years, and were mildly hypertensive (139/79 mm Hg) and overweight (body mass index 27 kg/m2). Of the 6247 patients not on dialysis, the mean eGFR was 27 mL/min/1.73 m2 and 80% had albuminuria. Women comprised 37% of the study population.
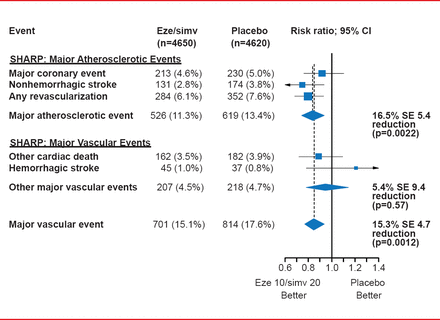
The primary outcome—major atherosclerotic events (coronary death, MI, nonhemorrhagic stroke, or any revascularization)—was significantly reduced with eze/simv compared with placebo (HR, 0.83; log-rank 2-sided p=0.0022) [Baigent C et al. Lancet 2011]. The events accrued at a very stable pace of about 3% to 4% per year.
Among the treatment group, consistent effects were seen across all primary and subsidiary outcome measures, including major vascular events (overall reduction of 15.3%; p=0.0012; Figure 1). Howevr, there was no effect on hemorrhagic stroke.
Results for the Primary Outcome and Major Vascular Events in SHARP
Eze/simv=ezetimibe 10 mg plus simvastatin 20 mg daily.
Reproduced from Baigent C et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377(9784):2181–2192. With permission from Elsevier.
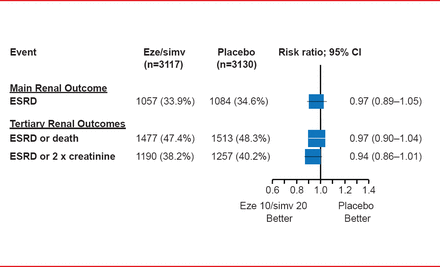
Eze/simv did not affect renal function, regardless of dialysis status, as measured by end-stage renal disease (ESRD), ESRD or death, or ESRD or a 2-fold increase in creatinine (Figure 2). No statistical heterogeneity was found between dialysis and nondialysis patients (p=0.25).
Renal Outcomes in the SHARP Study
Eze/simv=ezetimibe 10 mg plus simvastatin 20 mg daily.
Reproduced from Baigent C et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377(9784):2181–2192. With permission from Elsevier.
There were no significant differences in safety outcomes between the two groups, and there were no signals of cancer (HR, 0.99; 95% CI, 0.87 to 1.13; log-rank 2-sided p=0.89).
Adherence to the eze/simv regimen was maintained by about two thirds of patients. The study investigators calculated that full adherence with eze/simv would reduce the risk of the primary outcome by 25%, avoiding 30 to 40 events for every 1000 patients treated over 5 years.
Dr. Giugliano noted that the results of the IMPROVE-IT study [NCT00202878], expected to be available in late 2014, should provide further evidence about the benefits of adding ezetimibe to a statin. The study is comparing eze/simv 10/40 mg and simvastatin 40 mg in patients with recent acute coronary syndrome.
- © 2013 MD Conference Express®