Summary
This article discusses the nomenclature of neuroendocrine tumors (NETs), according to the updated 2010 World Health Organization (WHO) classification. This scheme classifies NETs based on prognostication by their proliferative activities, with respect to mitotic rate and Ki-67 labeling index. Since this accounts for tumor grading and prognosis, it facilitates development of treatment strategies, with somatostatin receptor 2a-positive tumors in particular demonstrating improved response to therapy with somatostatin analogs.
- Neuroendocrine Tumors
- Diabetes & Endocrinology Guidelines
- Neuroendocrine Tumors
- Diabetes & Endocrinology Guidelines
- Endocrinology
- Diabetes & Metabolic Syndrome
Robert Yoshiyuki Osamura, MD, PhD, International University of Health and Welfare, Tokyo, Japan, discussed the nomenclature of neuroendocrine tumors (NETs), according to the updated 2010 World Health Organization (WHO) classification. This scheme classifies NETs based on prognostication by their proliferative activities, with respect to mitotic rate and Ki-67 labeling index. Since this accounts for tumor grading and prognosis, it facilitates development of treatment strategies, with somatostatin receptor (SSTR) 2a-positive tumors in particular demonstrating improved response to therapy with somatostatin analogs (SAs).
NETs constitute a heterogeneous group of neoplasms considered to originate from a common precursor cell population found in endocrine glands, neuroendocrine tissues, pancreatic endocrine islets scattered between exocrine cells within glandular tissue. Tumors in this group include insulinoma, glucagonoma, and historically used misnomer “carcinoid.” Yet, although NETs have a wide spectrum of malignancy, these terminologies are somewhat problematic because they do not adequately convey the potential for malignant behavior that is associated with many of these tumors. Thus, NETs now are subdivided into NET G1, NET G2 and NEC.
Accordingly, in 2010, however, a new WHO classification was established, that is more definitive than its earlier counterparts, no longer uses the terminology “carcinoid,” and has prognostic and predictive value (Table 1). In this scheme, gastrointestinal and pancreatic tumors of endocrine and neuroendocrine origin are designated as gastroenteropancreatic neuroendocrine neoplasms (NENs), and are further subdivided into NETs (well-differentiated NENs) and neuroendocrine carcinomas (NECs; poorly differentiated NENs). NETs are further classified as grade Grade 1 (G1) or G2 tumors, according to their proliferative activity with respect to mitotic rate and Ki-67 labeling index (Table 2).
WHO Classification
Ki67 Index
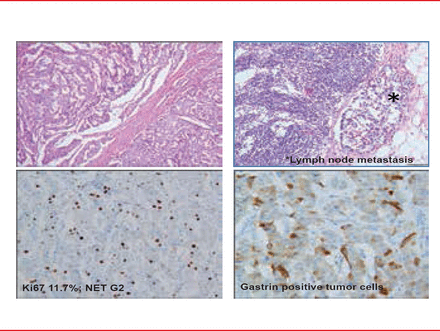
Mixed adenoneuroendocrine carcinoma, and hyperplastic and preneoplastic lesions comprise additional special groups. Figure 1 indicates a pancreatic NET G2 with gastric production.
Pancreatic Tumor NET G2 With Lymph Node Metasasis
Reproduced with permission from RY Osamura, MD, PhD.
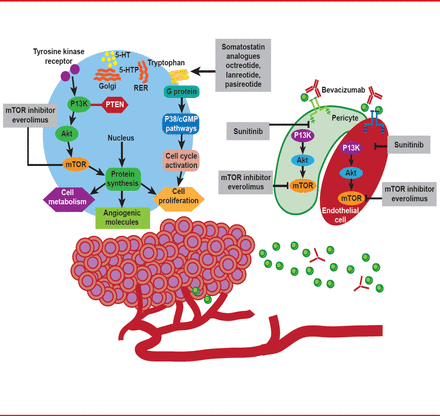
Although historically a difficult group of tumors to treat, in the current era of molecular targeted therapy, emerging novel strategies now hold promise for managing patients with advanced NETs (Figure 2) [Dong M et al. Clin Cancer Res 2012].
Various neuroendocrine markers and hormonal receptors with diagnostic, prognostic, and therapeutic implications have been described in NENs. These include SSTRs that mediate intracellular signaling pathways in cell proliferation and hormone secretion.
Strategies for Treatment of Patients With NETs
Reproduced from Dong M et al. New Strategies for Advanced Neuroendocrine Tumors in the Era of Targeted Therapy. Clin Cancer Res 2012; 18(7):1830–1836. With permission from the American Association of Cancer Research.
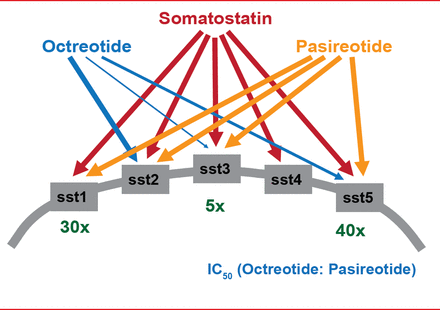
Immunohistochemical profiling of various SSTR subtypes in NETs now represents part of the routine diagnostic assessment of these tumors, serving as a useful predictor of responsiveness to various SAs, such as octreotide and pasireotide. These have been shown to block different SSTRs (SSTR1 to SSTR5; Figure 3), reduce the release of bioactive peptides and neuroamines, and delay NET growth. The multitargeted tyrosine kinase inhibitor sunitinib [Raymond E et al. N Engl J Med 2011], as well as the mammalian target of rapamycin (mTOR) inhibitor everolimus [Yao JC et al. N Engl J Med 2011], have also been demonstrated to improve progression-free survival in pancreatic NETs, confirming the importance of the phosphoinositide 3-kinase (PI3K)/Akt/mTOR pathway and angiogenesis in tumorigenesis, as well as important targets for further therapeutic advances [Dong M et al. Clin Cancer Res 2012]. It is also proposed that SSTR2a-positive NECs with NET histological features could be classified as NET G3, and may also respond to octreotide therapy.
Binding Affinities of Somatostatin and Its Analogs to Somatostatin Receptors
Reproduced with permission from RY Osamura, MD, PhD.
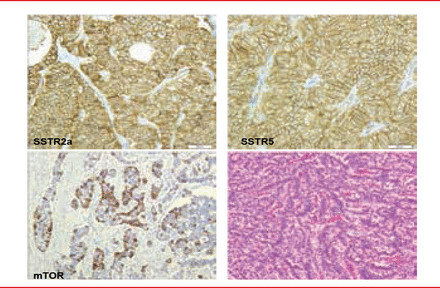
Figure 4 reveals a pancreatic NET with positive SSTR2a, SSTR5, and mTOR which may suggest various therapeutic possibiiies. Ki67 index is 11.7% and had a metastasis in a lymph node.
Pancreatic NET Positive for SSTR2a, SSTR5, and mTOR
Reproduced with permission from RY Osamura, MD, PhD.
Prof. Osamura therefore concluded that the WHO 2010 classification scheme should be recommended for diagnosis of pancreatic and gastrointestinal NETs G1 and G2, and NECs, and that since it incorporates with grading and staging, it provides a useful basis for prognostic prediction and treatment stratification.
- © 2013 MD Conference Express®