Summary
Hypoparathyroidism is a rare endocrine disorder with a wide range of etiologies, including surgery and radioactive iodine therapy. It can be acquired or genetic; curable or permanent. This article describes the differential diagnosis of the disease, summarizes the latest knowledge about the various causes of acquired hypoparathyroidism, and reviews recognized genetic mutations. The article also discusses skeletal and nonskeletal hypoparathyroidism.
- Thyroid Disorders
- Metabolic Bone Disease
- Endocrinology
- Diabetes & Metabolic Syndrome
- Thyroid Disorders
- Metabolic Bone Disease
Hypoparathyroidism is a rare endocrine disorder with a wide range of etiologies, including surgery and radioactive iodine therapy. It can be acquired or genetic; curable or permanent. Bart L. Clarke, MD, Mayo Clinic, Rochester, Minnesota, USA, described the differential diagnosis of the disease, summarized the latest knowledge about the various causes of acquired hypoparathyroidism, and briefly reviewed recognized genetic mutations.
The most common cause of chronic (>6 months) and acute (≤6 months) acquired hypoparathyroidism is iatrogenic in the setting of anterior neck surgery [Al-Azem H, Khan AA. Best Pract Res Clin Endocrinol Metab 2012]. Postsurgical hypoparathyroidism, said Dr. Clarke, is usually due to inadvertent removal of, or damage to, parathyroid glands or their blood supply.
Although neck surgery on the thyroid or parathyroid glands or major surgery for head and neck cancer are the most likely causes, hypoparathryoidism can also occur due to congenital or acquired disorders; autoimmune diseases; genetic abnormalities; and destruction or infiltrative disorders of the parathyroids [Al-Azem H, Khan AA. Best Pract Res Clin Endocrinol Metab 2012]. Impaired secretion of parathyroid hormone (PTH) may also be seen with hypomagnesemia or hypermagnesemia.
Except for autoimmune polyglandular syndrome type 1, the parathyroid glands are an infrequent target for autoimmunity [Brown EM. Endocrinol Metab Clin North Am 2009]. However, antibodies directed against the parathyroid cell surface calcium-sensing receptor have been identified in the serum of patients with autoimmune hypoparathyroidism.
The disease is challenging and multifactorial, with profound effects on the human skeleton [Bilezikian JP et al. J Bone Miner Res 2011]. Mishaela R. Rubin, MD, Columbia University, College of Physicians and Surgeons, New York, New York, USA, presented information on the clinical presentation of skeletal and nonskeletal hypoparathyroidism.
PTH is a key regulator of the bone remodeling rate. A reduction or absence of circulating PTH initially leads to a decrease in bone resorption, followed by a coupled reduction in bone formation [Bilezikian JP et al. J Bone Miner Res 2011].
The typical biochemical constellation in untreated hypoparathyroidism includes low circulating PTH levels, hyperphosphatemia, hypocalcemia, relatively high urinary calcium excretion, and reduced levels of 1,25-dihydroxyvitamin D [Rubin MR et al. J Bone Miner Res 2008]. Nonskeletal features include renal, biochemical and neuropsychological effects, along with extraskeletal calcifications. The skeletal features are structural and dynamic.
Renal compromise is common. In a long-term follow-up of patients with hypoparathyroidism, Mitchell et al. [J Clin Endocrinol Metab 2012] showed that of patients with 24-hour urine collection for calcium (n=53), 38% had at least one measurement >300 mg/day. Of those with renal imaging (n=54), 31% had renal calcifications. Rates of chronic kidney disease stage 3 or higher were 2- to 17-fold greater than age-appropriate norms.
Hypoparathyroidism is also associated with extraskeletal calcifications. Mitchell et al. [J Clin Endocrinol Metab 2012] found that 52% of individuals with head imaging (n=31) had basal ganglia calcifications. These may be related, in some way, to reduced quality of life (QoL) that patients with the disease often suffer [Cusano NE et al. J Clin Endocrinol Metab 2013].
In a recent study, individuals with hypoparathyroidism completed the RAND 36-item Health Survey, a measure of health-related QoL that covers eight domains of physical and mental health. At baseline, participants' scores were significantly lower than the normative reference range in all eight domains (scores −1.35 to −0.78; p<0.001 for all) [Cusano NE et al. J Clin Endocrinol Metab 2013].
Manifestations of hypoparathyroidism are not fully addressed by conventional treatment with calcium and vitamin D. The condition is the only classic hormone deficiency state for which there is no approved hormone replacement treatment [Mazziotti G et al. Endocrine 2012].
Nonetheless, new therapeutic options have become available, including two formulations of PTH: teriparatide [human PTH(1–34)] and the full-length molecule, PTH(1–84) [Cusano NE et al. Endocrine 2012]. Aliya A. Khan, MD, McMaster University, Hamilton, Ontario, Canada, reviewed the impact of PTH therapy on the disease.
Both treatments lower supplemental vitamin D requirements and increase markers of bone turnover. The pharmacokinetics of PTH(1–84) are substantially slower than those for PTH(1–34), which may help explain why dosing with the latter calls for multiple injections per day versus single or every other day dosing with PTH(1–84) [Cusano NE et al. J Clin Endocrinol Metab 2013].
In a study of PTH(1–84), Rubin et al. [J Bone Miner Res 2011] reported that subcutaneous administration reversed abnormal bone remodeling dynamics and structure, with bone turnover markers peaking at 5 to 9 months. In a prospective 4-year safety and efficacy study, Cusano et al. [J Clin Endocrinol Metab 2013] found that PTH(1–84) treatment improved biochemical control as well as mental and physical functioning.
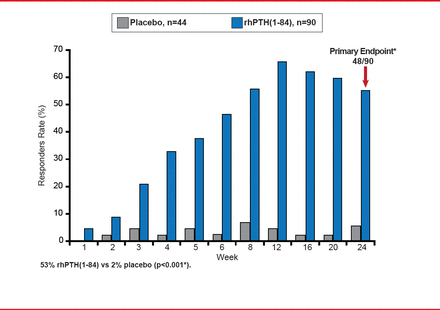
The Use of NPSP558 in the Treatment of Hypoparathyroidism study [REPLACE] is the first and largest randomized, double-blind, placebo-controlled, Phase 3 multinational trial to investigate the use of rhPTH(1–84), a recombinant human parathyroid hormone, for the treatment of adults with hypoparathyroidism [Oral Presentation at the Annual Meeting of The Endocrine Society 2012: June 23–26,2012. Houston TX]. It randomized 134 patients to rhPTH(1–84) or placebo. The primary endpoint was ≥50% reduction in oral calcium and active vitamin D while maintaining Ca(c) at Week 24.
Outcomes showed a significant difference in favor of rhPTH(1–84): 53% versus 2% placebo (p<0.001; Figure 1). The adverse event rate was similar with rhPTH(1–84) and placebo (90% vs 96%, respectively), with a lower discontinuation rate (7% vs 16%, respectively).
Current approaches to the management of hyperparathyroidism are based on severity, acuity, and underlying cause. The goals of accepted practice algorithms are to minimize risk and treat symptoms using large doses of calcium and activated vitamin D—a strategy that can cause ills that range from nephrocalcinosis and renal failure to cognitive dysfunction or brain fog [Bilezikian JP et al. J Bone Miner Res 2011].
REPLACE Study Primary Endpoint: Responder Rate at Week 24
Source: Bilezikian JP. ENDO 2012, Houston, TX. Abstract Number: S18–3.
The REPLACE study, the largest randomized controlled trial to date on rhPTH(1–84), offers not only hope, but also great promise of a more effective therapeutic option, with more to follow.
- © 2013 MD Conference Express®