Summary
Obesity drugs have eluded the United States Food and Drug Administration (FDA) approval process for 13 years, but with mechanisms of action identified and obesity recognized as a disease, that is changing. This article reviews the history of obesity pharmacology, present options in obesity pharmacotherapy, as well as the discovery of peptide-based mixed agonists based on glucagon.
- Obesity
- Endocrinology
- Diabetes & Metabolic Syndrome
- Obesity
Obesity drugs have eluded the United States Food and Drug Administration (FDA) approval process for 13 years, but with mechanisms of action identified and obesity recognized as a disease, that is changing. Caroline M. Apovian, MD, Boston University School of Medicine, Boston, Massachusetts, USA, reviewed the history of obesity pharmacology.
While there are more than 200 medications to treat hypertension and over 100 drugs for diabetes, those who suffer from obesity have few choices (Table 1). One reason is safety, which is of paramount importance due to the need for long-term management. Another is the number of people needing obesity treatment: 33% of American adults were considered obese (body mass index [BMI]≥30 kg/m2) in 2009 to 2010 [Ogden CL et al. NCHS Data Brief Number 82 2012].
Obesity Medications Available Prior to 2012
A third factor is the time and cost involved in the FDA approval process. It takes an average of 10 to 15 years for an experimental drug to travel from the lab to patients; 5 in 5000 compounds that enter preclinical testing make it to human testing; and it costs a company approximately $802 million to bring a new medicine to the market [How New Drugs Move through the Development and Approval Process. Tufts Center for the Study of Drug Development, Nov 2001].
Since 1997, four obesity medications—fenfluramine, dexfenfluramine, rimonabant, and sibutramine—have been withdrawn from the market for reasons that range from heart valve damage and pulmonary hypertension to increased risk of heart attack and stroke in high-risk cardiac patients [Kang JG, Park CY. Diabetes Metab J 2012].
There are three newcomers, all originally denied FDA approval, that have struggled to gain approval. The FDA rejected lorcaserin in October 2010 citing potential cancer risks. These were addressed, and the drug has been available in the United States since June 2013. Qsymia, a combination of phentermine and topiramate, was rejected by the FDA in 2010 due to numerous side effects, including raised heart rate, psychiatric problems, and a potential for birth defects. Ultimately, the FDA approved it in 2012 due to impressive weight loss results and commitments to a postmarketing program to mitigate these risks. Contrave, a combination of buproprion and naltrexone, is pending approval based on the results of a cardiovascular outcomes trial.
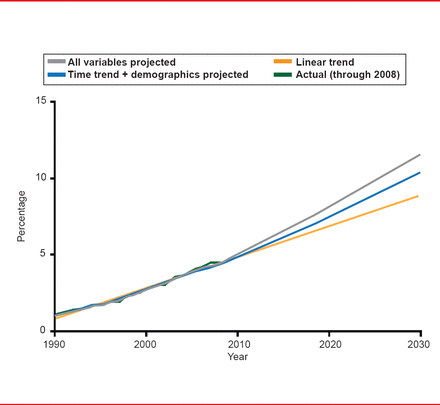
The need for new ways to treat obesity is critical. By 2030, an estimated 10% of Americans will have a BMI >40 kg/m2 (Figure 1) [Finkelstein EA et al. Am J Prev Med 2012]. Steven R. Smith, MD, Sanford Burnham Medical Research Institute, Orlando, Florida, USA, reviewed present options in obesity pharmacotherapy to address this dangerous trend.
Actual and Predicted Prevalence of Severe Obesity (BMI ≥40kg/m2)
Reproduced from Finkelstein EA et al. Obesity and Severe Obesity Forecasts Through 2030. Am J Prev Med 2012;42(6):563–570. With permission from Elsevier.
Although other anthropometric measures (eg, waist circumference, and waist-to-hip-ratio) could add extra information to patient workups, BMI, in and of itself, is a strong predictor of overall mortality both above and below the apparent optimum of approximately 22.5 to 25.0 kg/m2. At 30 to 35 kg/m2, median survival is reduced by 2 to 4 years; at 40 to 45 kg/m2, the figure is 8 to 10 years, which is comparable to the effects of smoking [Whitlock G et al. Lancet 2009].
Lorcaserin is a selective 5-HT2C receptor agonist that increases satiety and reduces food intake at a dose of 10 mg BID [Martin C. Obesity 2011]. It is indicated as an adjunct to a reduced calorie diet and increased physical activity for chronic weight management in adults with an initial BMI of 30 kg/m2 or 27 kg/m2 in the presence of ≥1 weight-related comorbid condition (eg, hypertension or type 2 diabetes).
Data from the BLOOM study show that at 1 year, 47.5% of patients in the lorcaserin group and 20.3% in the placebo group had lost ≥5% of their body weight (p<0.001), corresponding to an average weight loss of 5.8±0.2 kg with lorcaserin and 2.2±0.1 kg with placebo (p<0.001) [Smith SR et al. N Engl J Med 2010]. In conjunction with behavioral modification, lorcaserin was associated with significant weight loss and improved maintenance of weight loss compared with placebo.
Findings from the BLOOM-DM study also found that lorcaserin was associated with a significant improvement in glycemic control in patients with type 2 diabetes: 50.4% of patients treated with lorcaserin versus 26.3% on placebo achieved an HbA1C <7.0% from a baseline mean of 8.1% (p<0.001) [O'Neil PM et al. Obesity (Silver Spring) 2012].
Some of today's obesity drugs are combinations of existing pharmaceuticals. Tomorrow's will most likely be derived from such novel sources as peptide-based mixed agonists and co-agonists. Richard D. DiMarchi, PhD, Indiana University, Bloomington, Indiana, USA, discussed the discovery of peptide-based mixed agonists based on glucagon, a hormone that when given alone stimulates an increase in blood concentration of glucose.
The glucagon-GLP-1 co-agonist hypothesis is that chronic glucagon action will decrease fat mass by increasing energy expenditure via the glucagon receptor; GLP-1 will decrease fat mass by reducing food intake via the GLP-1 receptor; a GLP-1/glucagon co-agonist might potently decrease fat mass by synergistically affecting both components via 2 receptors; and a GLP-1/glucagon co-agonist should minimize the diabetogenic risk of a pure glucagon analog.
Preclinical testing in rodents and non-human primates of glucagon-based single molecule co-agonists showed high potency, balanced activity that was enhanced compared with pure GLP-1 agonists. The peptides decreased body weight and fat mass; improved blood glucose and insulin; and reduced blood lipid and liver fat content. Human clinical study is ongoing.
Other physiologically active components of interest include estrogen, incretins and adipokines (peptide/protein combination treatment), leptin, and extendin-4. To date, GLP-1 agonists provide significant clinical benefits, and glucagon/GLP-1 and GIP/GLP-1 co-agonists deliver significantly greater activity than GLP-1 in animal studies. The addition of leptin or estrogen provides additional efficacy and broadens the therapeutic index.
The door to medications for the treatment of obesity and metabolic disorders has opened just slightly, enough to let a few drugs through. However, great promise exists in the work on peptide-based mixed agonists.
- © 2013 MD Conference Express®