Summary
Teriparatide is absorbed across the nasal mucosa in two animal models, paving the way for a Phase 1 clinical trial to begin in late 2013. This article presents preclinical data of nanoenabled intranasal teriparatide for the treatment of osteoporosis.
- Diabetes & Endocrinology Clinical Trials
- Metabolic Bone Disease
- Hormone Therapy
- Diabetes & Endocrinology Clinical Trials
- Metabolic Bone Disease
- Hormone Therapy
- Endocrinology
- Diabetes & Metabolic Syndrome
Teriparatide is absorbed across the nasal mucosa in two animal models, paving the way for a Phase 1 clinical trial to begin in late 2013. Faron Jordan, PhD, Critical Pharmaceuticals, Nottingham, United Kingdom, presented preclinical data of nanoenabled intranasal teriparatide for the treatment of osteoporosis.
Administration of parathyroid hormone (PTH) is effective in promoting bone formation, resulting in increased bone mineral density, in patients with osteoporosis [Hodsman AB et al. Endocr Rev 2005]. Teriparatide is a peptide containing recombinant human PTH and is currently administered by daily injection. Intranasal delivery of teriparatide would be an attractive alternative to daily injections; however, peptide absorption across the nasal mucosa is limited [Illum L et al. J Control Release 2012]. The purpose of this preclinical study is to demonstrate that the CriticalSorb™ nasal delivery system developed by Critical Pharmaceuticals promotes the absorption of teriparatide and to establish the bioavailability of lead formulations prior to the initiation of a Phase 1 clinical trial.
In the preclinical study, 80 μg/kg of the teriparatide formulations were administered intranasally, using a narrow tipped pipette, to 4 conscious Sprague Dawley rats. Conscious New Zealand white female rabbits received 67 μg/kg of the lead teriparatide formulations. In both populations, subsequent subcutaneous injections were administered to allow relative bioavailability measurements. Following drug administration, blood was drawn for up to 6 hours and analyzed by liquid chromatography mass spectrometry/mass spectrometry. The bioactivity of the teriparatide CriticalSorb formulations were evaluated in a human osteoblast-like Saos-2 cell culture system.
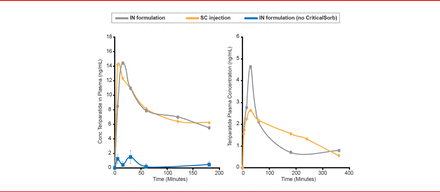
The bioavailability of the teriparatide/CriticalSorb formulation was 79% of subcutaneous teriparatide injection in rats (Figure 1). Intranasal administration of teriparatide without CriticalSorb resulted in very low levels of absorption. In rabbits, the lead formulation of teriparatide and CriticalSorb resulted in a bioavailability of 64% of the subcutaneous injection of teriparatide.
In the in vitro assay system, the cyclic adenosine monophosphate (cAMP) response to the teriparatide/CriticalSorb formulations was 113% greater than that of PTH 10 nm alone and PTH 10 nm in formulation with sodium hyaluronate, poloxamer 407, or chitosan (p<0.05). CriticalSorb without teriparatide did not elicit a cAMP response.
Bioavailability of Intranasal Teriparatide/CriticalSorb Formulations in Animal Models
IN=intranasal; SC=subcutaneous.
Reproduced with permission from F Jordan, PhD.
In the preclinical study, the stability of teriparatide was optimal with the addition of 5% w/v mannitol and 3% w/v of methionine at 37°C. The stability of teriparatide in this solution has remained stable at 12 weeks and long-term testing is currently underway.
The anticipated Phase 1 trial will have a 5-way crossover design to evaluate the safety of the teriparatide/CriticalSorb lead formulation compared with subcutaneous injection of teriparatide in postmenopausal healthy women. The primary endpoint of the Phase 1 trial will be to establish the pharmacokinetics of teriparatide following intranasal delivery. Currently, three doses of the teriparatide/CriticalSorb formulation are planned.
Dr. Jordan stated that the data from the preclinical study suggest that the intranasal delivery of teriparatide is absorbed across the nasal mucosa in two animal models. The Phase 1 trial is expected to begin at the end of 2013, with results expected in early 2014.
- © 2013 MD Conference Express®