Summary
The new oral anticoagulants have delivered promising results in terms of efficacy and safety. However, the data from the many clinical trials that have evaluated the new agents has provided physicians with a wealth of information for future drug development. This article presents five lessons learned from the recent clinical trials that evaluated the novel oral anticoagulant agents, including dabigatran, rivaroxaban, and apixaban.
- Thrombotic Disorders
- Thrombophilia
- Coagulation Defects
- Purpurea
- Other Hemorrhagic Conditions
- Thrombotic Disorders
- Thrombophilia
- Hematology
- Coagulation Defects
- Purpurea
- Other Hemorrhagic Conditions
The new oral anticoagulants (NOACs) have delivered promising results in terms of efficacy and safety. However, the data from the many clinical trials that have evaluated the new agents has provided physicians with a wealth of information for future drug development. John Eikelboom, MD, McMaster University Hamilton, Ontario, Canada, presented five lessons learned from the recent clinical trials that evaluated the novel oral anticoagulant agents, including dabigatran, rivaroxaban, and apixaban.
The first anticoagulant, hirudin, was introduced over 100 years ago, followed by heparin in 1939 and vitamin K antagonists in 1941. The anticoagulants argatroban (1990), bivalirudin (2000), and fondaparinux (2001) entered the clinic later. The most recent, novel oral anticoagulants, released in the late 2000s, include dabigatran, rivaroxaban, and apixaban, and were developed by an intelligent approach to drug design, making them selective for a single coagulant protein. Dr. Eikelboom pointed out that the Phase 3 trials for the new anticoagulants resulted in a large amount of data that created a good foundation on which to base recommendations.
Dr. Eikelboom stated that the unexpected findings from the Phase 3 trials of the new anticoagulants provided more unexpected information about thrombosis and hemostasis, and combined with the mechanism of action of the new agents, resulted in careful dose finding. From these trials, Dr. Eikelboom highlighted five key lessons.
The first lesson is that increasing the intensity of anticoagulant treatment has different effects on thrombosis and bleeding. Although a greater inhibition of thrombin occurs as anticoagulant treatment intensifies in the prevention of ischemic events, this eventually plateaus and a nonresponse is reached. However, bleeding does not appear to have a plateau effect. An example of this phenomenon occurred in the OASIS-5 trial, in which 25,000 patients with acute coronary syndrome were treated for 9 days with enoxaparin 1 mg/kg BID or fondaparinux 2.5 mg QD [The Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators. N Engl J Med 2006]. Although there was no significant difference in efficacy between the two agents, treatment with enoxaparin resulted in a significant increase in risk of bleeding of ∼2-fold compared with fondaparinux. Dr. Eikelboom suggested that the difference in bleeding may be due to the different mechanisms of action, or due to the differing intensity of treatment. A subsequent study demonstrated that at 6 hours post dose, the antifactor Xa effect was greater in patients that received enoxaparin, whereas the thrombin generation was greater in patients that received fondaparinux [Anderson JAM et al. J Thromb Haemost 2010].
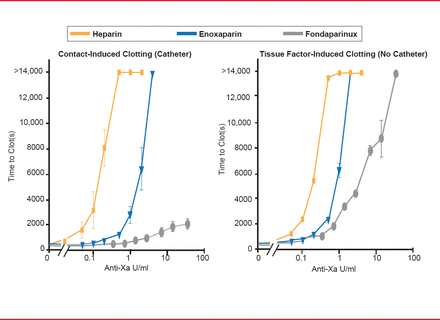
The second lesson is that the efficacy of the anticoagulant is dependent on the stimulus that is causing thrombosis. The two triggers of coagulation include the exposure of blood to tissue factor due to injury (extrinsic pathway) and the contact pathway (intrinsic pathway). The contact pathway is initiated when blood is exposed to artificial surfaces, such as the tubing of a catheter. For example, in the OASIS-5 trial, fondaparinux was demonstrated to have lower efficacy in preventing catheter thrombosis than enoxaparin [The Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators. N Engl J Med 2006]. Another study evaluated the effect of heparin, enoxaparin, and fondaparinux based on the clotting stimulus [Yau JW et al. Blood 2011]. The presence of a catheter induces contact activation; fondaparinux was demonstrated to have little efficacy compared with heparin and enoxaparin (Figure 1). Without a catheter, the injury pathway is induced and the efficacy of fondaparinux increases dramatically. Dr. Eikelboom pointed out that this difference in efficacy is most likely due to their different mechanism of actions, in particular, their unique molecular targets.
Anticoagulation Efficacy Differs by Clotting Stimulus
Reproduced from Yau JW et al. Mechanism of catheter thrombosis: comparison of the antithrombotic activities of fondaparinux, enoxaparin, and heparin in vitro and in vivo. Blood 2011;118(25)6667–6674. With permission from the American Society of Hematology.
The third lesson is that the anticoagulant therapy-associated risk of bleeding is specific to the vascular bed or organ. Dr. Eikelboom highlighted that oral anticoagulants are associated with greater gastrointestinal bleeding and less intracranial bleeding, while vitamin K antagonists like warfarin are associated with greater intracranial bleeding and fewer gastrointestinal events. Again, the reason for this maybe due to differences in mechanism of action. For example, dabigatran directly inhibits thrombin. Therefore, in areas where a large amount of thrombin is produced, it is possible that the high thrombin concentration can overwhelm the concentration of dabigatran. In contrast, warfarin inhibits the formation of thrombin and is thus not overwhelmed in areas where large amounts of thrombin are potentially produced.
The fourth lesson is that the inhibition of thrombin appears to be a less effective mechanism for protection against myocardial infarction (MI). Similar to the mechanism behind the risk of bleeding mentioned above, it appears that the therapeutic target of the drug underlies the difference in protection against MI. Dr. Eikelboom presented the example of a ruptured plaque, which leads to thrombin formation. Dabigatran directly inhibits thrombin, and if a large amount of thrombin is produced, dabigatran can be overwhelmed. In contrast, warfarin targets the formation of thrombin, so less thrombin is produced overall, thus leading to a greater protection against MI. However, Dr. Eikelboom pointed out that anti-Xa inhibitors are less likely to be overwhelmed because factor Xa is produced in lower concentrations than thrombin.
The fifth lesson is that in atrial fibrillation (AF) and venous thromboembolism (VTE) treatment, the dose selection is dependent on dose-finding studies that were performed in patients with deep vein thrombosis. Dr. Eikelboom highlighted that in a collective eight Phase 2 trials of the NOACs for VTE prevention and VTE treatment, over 5000 patients and 600 thrombi, as well as over 1000 patients and 300 thrombi were evaluated, respectively. In contrast, only one Phase 2 trial with about 500 patients and 2 thrombi was evaluated for the treatment of AF. Therefore, it is clear that the dose used in the Phase 3 trials for AF were not only based on the Phase 2 trial for the AF indication, but information from other trials must have been used.
Intelligent drug design has resulted in several NOACs that have demonstrated both efficacy and safety. However, Dr. Eikelboom opined that the many clinical trials that have been conducted to evaluate the NOACs have provided several important lessons for future drug development.
- © 2013 MD Conference Express®