Summary
In a nonhuman primate model of hemolytic uremic syndrome, complement was not activated despite clear microvascular thrombosis and cellular injury. Complement is an important immune defense mechanism. This article presents outcomes from a study on the role of complement in HUS and thrombotic microangiography induced by Escherichia coli (E. coli) Shiga toxins.
- Hematology Clinical Trials
- Anemias
- Bacterial Infections
- Hematology Clinical Trials
- Hematology
- Anemias
- Bacterial Infections
In a nonhuman primate model of hemolytic uremic syndrome (HUS), complement was not activated despite clear microvascular thrombosis and cellular injury. Complement is an important immune defense mechanism. Shinichiro Kurosawa, MD, PhD, Boston University School of Medicine, Boston, Massachusetts, USA, presented outcomes from a study on the role of complement in HUS and thrombotic microangiography induced by Escherichia coli (E. coli) Shiga toxins.
Enterohemorrhagic Shiga toxin-producing E. coli (EHEC), the leading cause of acute renal failure in otherwise healthy children, is associated with the potentially lethal complication of HUS. EHEC are food- and water-borne bacteria, contributing to the estimated 76 million illnesses, 325,000 hospitalizations, and 5200 deaths each year in the United States attributable to foodborne outbreaks, with a total annual cost of $10 to $83 billion [Bavaro MF. Curr Gastroenterol Rep 2012]. Toxins from these bacteria cause kidney, intestinal, and neurologic damage.
A 2011 outbreak of an E. coli strain secreting Shiga toxin type-2 in Germany infected 3842 individuals, many after consuming contaminated fenugreek sprouts. More than 50% of them required hospitalization; 855 developed HUS; and 54 died [Werber D et al. BMC Med 2012].
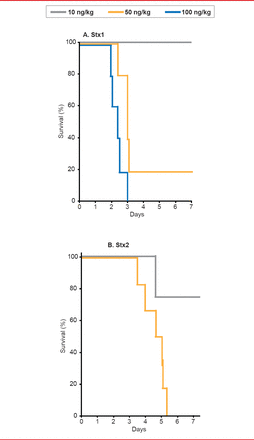
In order to develop a clinically-relevant animal model of HUS, Stearns-Kurosawa and colleagues [Infect Immun 2010] studied the effects of Shiga toxin types 1 and 2 (Stx1, Stx2) in nonhuman primates, comparing the in vivo consequences of the toxins in a parallel and reproducible manner. They found that the time course, pathology, and cytokine profiles differed between Stx1 and Stx 2, but that both induced HUS (Figure 1).
Time Course, Pathology, and Cytokine Profiles Differ Between Stx1 and Stx2
Reproduced with permission from S Kurosawa, MD, PhD.
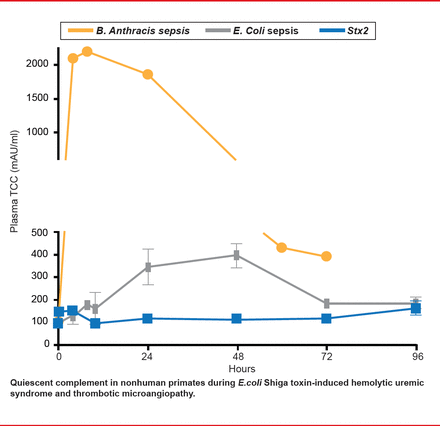
In a subsequent study, Lee and colleagues [Blood 2013] asked whether complement activation is a major pathway for HUS development in this animal model. Some patients show evidence of complement activation during EHEC infection, raising the possibility of therapeutic targeting of complement for relief. Nonhuman primate models indicate otherwise. They found that platelet levels declined in a dose-dependent manner after Stx1 and Stx2 (thrombocytopenia). Evidence of coagulation and full HUS development was clear. D-dimer was elevated, indicating that both coagulation and fibrinolysis took place. Damage-associated molecular patterns (DAMPs; HMGB1 and histones) were also elevated, indicating tissue damage. However, complement was not activated. There were no significant increases in soluble terminal complement complex (C5b-9) levels after challenge with lethal Stx1 (n=6) or Stx2 (n=5) in plasma samples from TO to euthanasia at 49.5 to 128 hours post challenge (Figure 2). This contrasts with robust complement activation in bacteria sepsis models (Bacillus anthracis, nontoxin E. coli), which have disseminated intravascular coagulation, rather than HUS.
Complement Activation Is Not the Major Pathway for HUS Development.
Reproduced with permission from S Kurosawa, MD, PhD.
These studies found that in preclinical models, complement activation is not required for the development of thrombotic microangiopathy and HUS induced by EHEC Shiga toxins. The global nature of food processing and distribution raises the stakes in the study of EHEC, heightening the imperative to recognize, treat, and report it. To date, no specific treatment is available.
- © 2013 MD Conference Express®