Summary
A new era in the treatment of hemophilia has emerged with a new generation of protein therapeutics. This article provides an overview of the future of coagulation factor replacement therapy. Protein engineering has increased the clinical potential and reduced rapid clearance of antihemophilic drugs from the body. Current therapy for hemophilia is safe and effective but immunogenic. Next generation antihemophilic drugs should have enhanced efficacy, greater safety, reduced immunogenicity, and improved delivery.
- Hematology Clinical Trials
- Hemorrhagic Disorders
- Hematology
- Hematology Clinical Trials
- Hemorrhagic Disorders
A new era in the treatment of hemophilia has emerged with a new generation of protein therapeutics. Flora Peyvandi, MD, PhD, University of Milan, Milan, Italy, provided an overview of the future of coagulation factor replacement therapy.
Protein engineering has increased the clinical potential and reduced rapid clearance of antihemophilic drugs from the body. Current therapy for hemophilia is safe and effective but immunogenic. Next generation antihemophilic drugs should have enhanced efficacy, greater safety, reduced immunogenicity, and improved delivery. Bio engineering technologies that have been applied successfully to other therapeutic proteins are now being applied to Factor VIII, Factor IX, and Factor Vila. These technologies include the addition of polyethylene glycol (PEG) polymers and polysialic acids, alternative formulations with PEG-modified liposomes (PEG-Lip), and fusion proteins technologies. Pegylation of a protein extends its half-life and increases drug efficacy.
Glycopegylation allows for targeted pegylation so that the PEG can be attached to specific parts of the coagulation factors, such as the B domain for Factor VIII and the activation peptide for Factor IX. Upon activation, the pegylated portion of the protein is cleaved off, leaving the native activated coagulation factor.
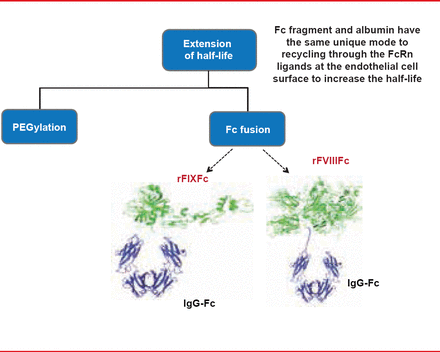
An alternate strategy to extend the half-life of proteins is to fuse them to another protein with a much longer half-life, such as the fragment crystallizable (Fc) region of an immunoglobulin (Figure 1). Fc-containing proteins that are internalized by endothelial cells bind to the neonatal Fc receptor (FcRn) present in the acidified endosome, and are recycled back to the cell surface and subsequently released back into plasma at physiologic pH. These approaches markedly increase molecular weight, which reduces renal clearance.
Half-Life Extension
Reproduced with permission from F Peyvandi, MD, PhD.
Albumin fusion technology yields an altered version of a protein by fusing the gene for human albumin to the gene that encodes the active protein drug. This technology increases the protein's molecular weight, prolonging the half-life in vivo. The albumin molecule also masks the protein, rendering it resistant to proteases and less immunogenic.
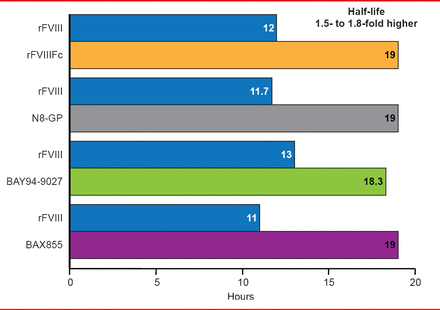
Modified long-acting recombinant Factor VIII products in late-phase clinical studies have half-lives of 1.5- to 1.6-fold longer than their unmodified versions (Figure 2). Similar strategies have been used to extend the half-life of Factor IX by 3- to 5-fold.
Half-Life Extension of Long Acting rFVIII
Reproduced with permission from F Peyvandi, MD, PhD.
Current prophylactic treatment requires infusion 2 to 3 times weekly using Factor VIII and 2 times weekly using FIX products. In the future, the frequency of administration will be significantly reduced to once or twice weekly and every 1 to 2 weeks with long-acting recombinant FVIII and FIX products respectively, said Prof. Peyvandi.
Bioengineering strategies have also been employed to extend the half-life of recombinant Factor Vila through site-specific pegylation, albumin fusion, or modification of amino acid sequence.
RNA interference is a cellular pathway of gene silencing in a sequence-specific manner at the mRNA level. A short interfering RNA, ALN-AT3, which employs a hepatocyte-targeting ligand, has been developed against antithrombin. In nonhuman primates, ALN-AT3 yielded potent and durable knockdown of antithrombin with an up to 4-fold increase in peak thrombin generation. Clinical trial using this novel drug will start at the end of this years.
Another approach is a humanized bispecific antibody to Factors IXa and X, termed hBS23 (ACE910), which is able to restore Factor VIII hemostatic activity. It is delivered via intravenous injection and has a 2-week half-life. Subcutaneous bioavailability of ACE910 is 84%. A clinical trial using this product has already started in Japan [JapicCTI-121934; http://www.clinicaltrials.jp].
- © 2013 MD Conference Express®