Summary
Multiple organizations have published clinical practice guidelines that recommend strategies for the management of atrial fibrillation (AF) in the clinic. This article presents various clinical practice recommendations for rate control in AF. Also discussed are the recommendations for antiarrhythmic therapy in AF, recommendations for antithrombotic therapy in AF, tools and techniques used in cryoballoon ablation of pulmonary veins, duty cycled radiofrequency ablation tools and techniques, as well as information about laser ablation.
- Arrhythmias
- Cardiology Guidelines
- Cardiology & Cardiovascular Medicine
Multiple organizations have published clinical practice guidelines that recommend strategies for the management of atrial fibrillation (AF) in the clinic. Anne M Gillis, MD, University of Calgary, Calgary, Alberta, Canada, presented various clinical practice recommendations for rate control in AF. The treatment goals for AF include ventricular rate and rhythm control, which should ultimately improve symptoms and patient outcomes [Gillis AM et al. Can J Cardiol 2011].
The Canadian Cardiovascular Society (CCS) Guidelines recommend that ventricular rate be assessed at rest and during exercise in all patients and that treatment should aim to maintain a resting heart rate of ≤100 bpm [Gillis AM et al. Can J Cardiol 2011]. The 2011 American College of Cardiology Foundation (ACCF)/American Heart Association (AHA)/Heart Rhythm Society (HRS) Guidelines state that aggressive rate control is not beneficial compared with a less aggressive strategy, particularly in patients with persistent AF with stable ventricular function [Anderson JL et al. Circulation 2013]. Patients that have uncontrollable ventricular rates during AF despite pharmacologic treatment are recommended to receive atrioventricular junction ablation with implantation of a pacemaker [Gillis AM et al. Can J Cardiol 2011].
John Camm, MD, St George's University, London, United Kingdom, presented the recommendations for antiarrhythmic therapy in AF. The European Heart Rhythm Association (EHRA) updated its guidelines in 2012, which addresses the use of pharmacological cardioversion with vernakalant and the use of oral antiarrhythmic therapy [Camm AJ et al. Eur Heart J 2012]. Vernakalant is an ion channel blocker that specifically targets the atria with little hemodynamic adverse effects [Fedida D et al. J Cardivasc Electrophysiol 2005]. In several clinical trials, the mean time to cardioversion was 8 to 14 minutes, with 75% to 80% of patients converted, following the first dose of vernakalant. Prof. Camm pointed out that treatment with vernakalant is associated with a greater risk of hypotension and ventricular arrhythmia, particularly in patients with congestive heart failure (CHF) [Kynapid NDA 22–034 Astellas Pharm US, Inc]. With these data, the EHRA Class I recommendations are to use pharmacologic cardioversion with intravenous flecainide, propanfenone, ibutilide, or vernakalant if there is little or no structural heart disease present [Camm AJ et al. Eur Heart J 2012]. Class IIb recommendations suggest that patients with moderate structural heart disease and AF for <7 days may receive intravenous vernakalant and in postoperative AF ≤3 days following cardiac surgery.
The EHRA Guidelines recommend the use of dronedarone in patients with recurrent AF for the maintenance of sinus rhythm, but do not recommend it in patients with permanent AF [Camm AJ et al. Eur Heart J 2012]. The 2012 update to the ACCF/AHA/HRS guidelines indicates that dronedarone can be used to decrease the need for hospitalization as a result of cardiovascular events in patients with paroxysmal AF or for persistent AF following cardioversion, but should not be given to patients with Class IV heart failure [Wann LS et al. J Am Coll Cardiol 2011].
The ESC Guidelines recommend that dronedarone should not be used in Class II or Class IV heart failure and only used with caution and as a last resort in mild to moderate heart failure or in patients with left ventricular systolic dysfunction [Camm AJ et al. Eur Heart J 2012].
Hugh Calkins, MD, Johns Hopkins University, Baltimore, Maryland, USA, describe the current recommendations for catheter ablation in AF. In the 2012 update to the HRS/EHRA/European Cardiac Arrhythmia Society (ECAS) Guidelines on catheter and surgical ablation of AF, indications for catheter ablation were identified as symptomatic AF that is refractory or intolerant to at least one Class 1 or 3 antiarrhythmic therapy and in symptomatic AF before beginning antiarrhythmic therapy with a Class 1 or 3 drug [Calkins H et al. Heart Rhythm 2012]. Prior to or immediately after the ablation procedure, heparin should be given, unless the patient already receives warfarin.
The 2012 update of the ESC Guidelines for catheter ablation indicates that, due to new data, catheter ablation can be recommended as first-line therapy for rhythm control in selected patients with AF [Camm AJ et al. Eur Heart J 2012]. Anticoagulant therapy is also recommended before or after an ablation procedure to reduce thromboembolic risk.
Gregory YH Lip, MD, University of Birmingham, Birmingham, United Kingdom, discussed the recommendations for antithrombotic therapy in AF. The 2012 recommendations of the American College of Chest Physicians (ACCP) suggest the use of oral anticoagulant therapy in patients with an CHADS2 score of ≥1, and where oral anticoagulant therapy is indicated, then dabigatran 150 mg twice daily could be considered (Grade IIb) [You JJ et al. Chest 2012]. In those with a CHADS2 score=0, other risk factors such as age 65 to 74 years, vascular disease and female gender may indicate the need for oral anticoagulation. In the absence of all risk factors, no antithrombotic therapy is recommended. The 2012 update by the CCS recommends oral anticoagulant therapy, preferably with dabigatran or rivaroxaban in patients at intermediate to high risk [Skanes AC et al. Can J Cardiol 2012]. In patients at low risk, no antithrombotic therapy is recommended, but with ≥1 stroke risk factors, oral anticoagulant therapy may also be considered. The ESC 2012 update for the management of AF strongly recommends that physicians focus on identifying “truly low-risk” patients with AF (age <65 years and lone AF [including females] or a CHA2DS2-VASc score=0) as the first decision step, rather than focusing on patients at high risk [Camm AJ et al. Eur Heart J 2012]. The CHA2DS2-VASc score is the only stroke risk score recommended in the ESC 2012 guideline. Patients with a CHA2DS2-VASc score of ≥2 should receive anticoagulant therapy (Class I recommendation), preferably with dabigatran, rivaroxaban, or apixaban. Oral anticoagulant therapy should be considered in patients with a CHA2DS2-VASc score=1 (Class IIa), with the exception of females who have a score=1 only on basis of their gender.
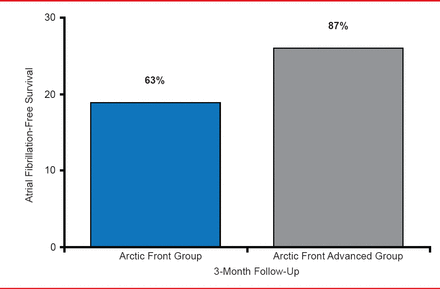
Thomas Neumann, MD, Kerckhoff Heart Center, Bad Nauheim, Germany, presented tools and techniques used in cryoballoon ablation of pulmonary veins. A circular mapping catheter with 8 electrodes can be introduced through the inner lumen of the shaft of cryoballoon catheter (Achieve catheter). A second generation of cryoballoon, called Arctic Front Advanced is an improvement over the previous generation (Arctic Front) because it provides more uniform and distal cooling and has an increased refrigerant flow. A single-center, nonrandomized study compared 30 patients treated with the 28-mm Arctic Front with a 300-second application with 30 patients treated with the 28-mm Arctic Front Advanced with a 240-second application time [Fürnkranz A et al. J Cardiovasc Electrophysiol 2013]. After the first 3 months following ablation, 37% of patients treated with Arctic Front and 13% of patients treated with Arctic Front Advanced experienced early AF recurrence. In addition, AF-free survival was greater in patients treated with Arctic Front Advanced compared with patients treated with Arctic Front (Figure 1).
Prof. Neumann explained several techniques that can be used with cryoballoon ablation, including hockey stick positioning, big loop positioning, inversed C-position, pull down maneuvers, and cross talk. Complications of cryoballoon therapy include phrenic nerve palsy, esophageal thermal lesions, and atrioesophageal fistula. However, Prof. Neumann suggested that some techniques may help prevent these complications.
Frank Halimi, MD, Hospital Privé Parly 2, Le Chesnay, France, discussed duty cycled radiofrequency ablation tools, techniques, and suggestions for preventing complications. The pulmonary vein ablation catheter (PVAC) is 25 mm with 10 electrodes of 3 mm length spaced 3 mm apart. The PVAC can have a single or multiple arrays. The PVAC user determines the energy mode, which can be unipolar or bipolar only, or various mixtures of both. Prof. Halimi highlighted that imaging is important to understand the patient's anatomy, which is critical in preventing complications such as right phrenic nerve injury.
AF-Free Survival Following Treatment With Cryoballoon Ablation
In the TTOP-AF trial [NCT00514735], 210 patients with persistent AF were randomized to receive medical management or cryoballoon ablation. At 6-month follow-up, 55.8% of patients treated with ablation were off pharmacologic therapy compared with 26.4% in the medical-management arm. The complication rate was 12.3% in the cryoballoon ablation arm and included 4 acute strokes, 1 stroke, and 6 pulmonary vein stenosis. Prof. Halimi pointed out that asymptomatic cerebral embolism is an issue with this technology, but suggested that the risk may be reduced with procedural changes.
Petr Neuzil, MD, PhD, Na Homolce Hospital, Prague, Czech Republic, presented information about laser ablation. This technology is visually guided with a compliant balloon with nine different sizes. In an international, multicenter, open-label study of 200 patients treated with visually guided laser ablation, 98.8% of pulmonary veins were isolated, with 79.4% isolated on the first attempt [Dukkipati SR et al. Circ Arrhythm Electrophysiol 2013]. Complications included phrenic nerve injury in 2.5% of patients, cardiac tamponade in 2% of patients, major bleeding in 1.5% of patients, and minor bleeding in 3.5% of patients.
Multiple organizations in Europe and the United States have recently updated their clinical practice guidelines for the management of AF. Although there is a large amount of similarity across the guidelines, there are some areas that slightly differ in recommendations.
- © 2013 MD Conference Express®