Summary
Infections associated with cardiovascular implantable electronic devices (CIEDs) cause substantial morbidity and mortality. The AIGISRx antibacterial envelope (ABE) for prophylaxis of CIED infections was approved by the Food and Drug Administration in 2008 for use with perioperative IV antibiotics. The AIGISRx Envelope for Prevention of Infection Following Replacement With an Implantable Cardioverter-Defibrillator (ICD) [CITADEL; NCT01043861] or Cardiac Resynchronization Therapy Device (CRT) [CENTURION; NCT01043705] trials prospectively evaluated the effectiveness of the ABE in patients implanted with a non-de novo single- or dual-chamber ICD (CITADEL) or non-de novo CRT (CENTURION) devices.
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Bacterial Infections
- Cardiology & Cardiovascular Medicine
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Bacterial Infections
Infections associated with cardiovascular implantable electronic devices (CIEDs) cause substantial morbidity and mortality. Although perioperative intravenous (IV) antibiotic prophylaxis is standard care for CIED procedures, the rate of CIED infections has been increasing. The AIGISRx antibacterial envelope (ABE) for prophylaxis of CIED infections was approved by the Food and Drug Administration (FDA) in 2008 for use with perioperative IV antibiotics. The ABE consists of a polypropylene mesh coated with a resorbable tyrosine polymer impregnated with rifampin and minocycline, which are released for 7 to 10 days.
The AIGISRx Envelope for Prevention of Infection Following Replacement With an Implantable Cardioverter-Defibrillator (ICD) [CITADEL; NCT01043861] or Cardiac Resynchronization Therapy Device (CRT) [CENTURION; NCT01043705] trials prospectively evaluated the effectiveness of the ABE in patients implanted with a non-de novo single- or dual-chamber ICD (CITADEL) or non-de novo CRT (CENTURION) devices. Charles A. Henrikson, MD, Oregon Health Sciences University, Portland, Oregon, USA, presented results of the 3-month interim analysis of these studies.
The study designs planned for ICD implantation in 2300 patients to be compared with published controls (CITADEL) and for CRT implantation in 2000 patients to be compared with a site- and case-matched retrospective control cohort (CENTURION). The endpoints were CIED infection and mechanical complications rates. Eligible patients underwent generator replacement with a single-or dual-chamber ICD or CRT and the ABE, with or without lead revision or addition. Data for the interim analysis were obtained from a total of 1000 patients in the CITADEL (n=403) and CENTURION (n=597) studies at the 3-month follow-up visit.
At 3 months, the major infection rate among all 1000 patients (CITADEL and CENTURION) was 0.10% (95% CI, 0.00 to 0.56), with one major infection in the ICD cohort and none in the CRT cohort. The minor infection rate was 1.00% (95% CI, 0.48 to 1.83), with 4 minor infections in the ICD cohort and 6 in the CRT cohort.
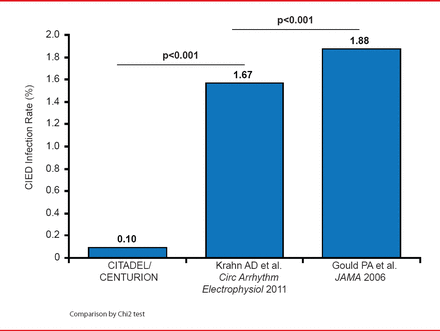
Comparison with published controls showed a significant reduction in infection rates among patients treated with the ABE (0.10%, CITADEL+CENTURION) versus controls 1.67% (p<0.001) [Krahn AD et al. Circ Arrhythm Electrophysiol 2011], 1.88% (p<0.001) [Gould PA et al. JAMA 2006] (Figure 1).
Comparison of Infection Rates in CITADEL Plus CENTURION Versus Published Controls
Reproduced with permission from CA Henrickson, MD.
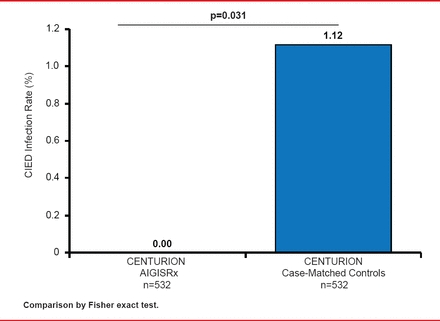
Among the 597 CENTURION patients, 532 had an eligible case-match. Major infections were reported in 0.00% (95% CI, 0.0 to 0.7) of ABE-treated patients versus 1.12% (95% CI, 0.4 to 2.4) in the case-matched controls (p=0.031; Figure 2). There was no significant difference in the rate of mechanical complications between the two groups.
The results of this interim analysis showed that use of the AIGISRx antibacterial envelope in patients undergoing CIED procedures significantly reduced the risk of infections when compared with published historical controls and case-matched controls. There was no increase in serious device-related complications among the CITADEL and CENTURION patients treated with the AIGISRx antibacterial envelope compared with controls.
Infection Rates With ABE Versus Case-Matched Controls
Reproduced with permission from CA Henrickson, MD.
- © 2013 MD Conference Express®