Summary
Patients with rheumatoid arthritis (RA) who transition directly from adalimumab to tofacitinib maintained a clinical response. This article presents results from the open-label extension Oral Rheumatoid Arthritis Phase 3 Trials Sequel study [ORAL; NCT00413699] of the randomized, double-blind, placebo-controlled ORAL Standard study in which the two treatments were compared in patients with active RA [NCT00853385].
- Rheumatology Clinical Trials
- Rheumatoid Arthritis
- Rheumatology Clinical Trials
- Rheumatoid Arthritis
- Rheumatology
Patients with rheumatoid arthritis (RA) who transition directly from adalimumab (ADA) to tofacitinib (TOFA) maintained a clinical response. Mark Genovese, MD, Stanford University, Stanford, California, USA, presented results from the open-label extension Oral Rheumatoid Arthritis Phase 3 Trials Sequel study [ORAL; NCT00413699] of the randomized, double-blind, placebo-controlled ORAL Standard study in which the two treatments were compared in patients with active RA [NCT00853385].
TOFA is a novel oral Janus kinase inhibitor that has been approved by the United States Food and Drug Administration for the treatment of RA. In ORAL Standard, in subjects with RA (n=717) receiving stable doses of methotrexate, the addition of TOFA 5 or 10 mg twice daily significantly performed better than placebo and was numerically similar to ADA 40 mg Q2W on three primary outcome measures: 20% improvement at Month 6 in the American College of Rheumatology scale (ACR20); the change from baseline to Month 3 in the score on the Health Assessment Questionnaire-Disability Index (HAQ-DI); and the percentage of patients at Month 6 who had a Disease Activity Score for 28-joint counts based on the erythrocyte sedimentation rate (DAS28–4[ESR]) of <2.6 [van Vollenhoven RF et al. N Engl J Med 2012].
Subjects who completed ORAL Standard were eligible to enroll in ORAL Sequel, receiving TOFA 10 mg BID, without a washout, with the timing of the first TOFA dose ≤1 week following the last dose of ADA in ORAL Standard. The goal was to define the efficacy and safety of transitioning from ADA to TOFA without a washout.
Of the 204 subjects randomized to ADA, 145 enrolled in ORAL Sequel and 125 of them began TOFA without a washout ≤1 week after their last dose of ADA in Oral Standard. Of the 201 subjects randomized to TOFA, 148 enrolled in ORAL Sequel and 124 took their first dose of TOFA ≤1 week after their last dose of TOFA in Oral Standard.
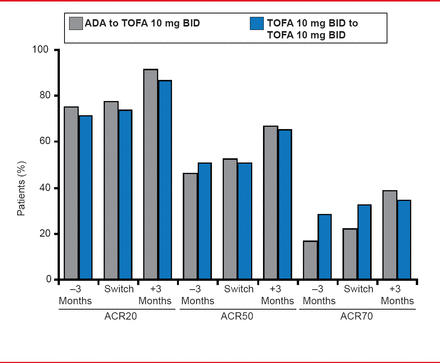
Results were reported for subjects randomized to ADA 3 months before the end of ORAL Standard, at the end of ORAL Standard, and 3 months after the transition to TOFA in ORAL Sequel. Data for TOFA 10 mg BID, during each phase were reported for the same time points. The ACR20 response rate in patients randomized to ADA were 74.2% at 3 months before the end of ORAL Standard, 76.6% at the end, and 90.5% at 3 months after the transition to TOFA in ORAL Sequel. A similar pattern was observed for ACR50 and ACR70 responses (Figure 1).
Efficacy: ACR Responses in Last 3 Months of ORAL Standard and First 3 Months of ORAL Sequel Study
Reproduced with permission from M Genovese, MD.
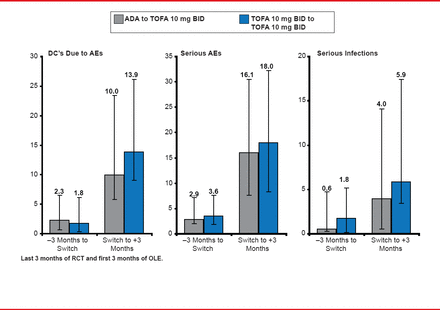
Mean change from baseline in HAQ-DI in patients transitioning from ADA to TOFA was −0.55, −0.60, and −0.70 at these same three time points. Efficacy results in patients who continued on TOFA were similar at the same time points and showed a similar pattern of increases from ORAL Standard to ORAL Sequel. The rate of serious adverse events increased post transition in both groups, when analyzed per 100 patient-years (Figure 2). The overlapping immunomodulatory effects of ADA and TOFA did not appear to be the cause of the increase in safety-related events, because they were increased in both groups.
Safety: Incidence Rates per 100 Patient-Years
Reproduced with permission from M Genovese, MD.
- © 2013 MD Conference Express®