Summary
To date, few direct comparisons of biologic agents for the treatment of rheumatoid arthritis (RA) have been conducted, resulting in a lack of evidence-based guidance for selection of biologic therapy. The Abatacept Versus Adalimumab Comparison in Biologic-Naïve RA Subjects With Background Methotrexate trial [AMPLE; NCT00929864] was the first powered head-to-head study comparing two biologic disease-modifying antirheumatic drugs, abatacept and adalimumab, with the standard of care, methotrexate. This article discusses Phase 3b of the AMPLE trial.
- Rheumatoid Arthritis
- Rheumatology Clinical Trials
- Rheumatoid Arthritis
- Rheumatology Clinical Trials
- Rheumatology
To date, few direct comparisons of biologic agents for the treatment of rheumatoid arthritis (RA) have been conducted, resulting in a lack of evidence-based guidance for selection of biologic therapy. The Abatacept Versus Adalimumab Comparison in Biologic-Naïve RA Subjects With Background Methotrexate trial [AMPLE; NCT00929864] was the first powered head-to-head study comparing two biologic disease-modifying antirheumatic drugs (DMARDs), abatacept (ABA) and adalimumab (ADA), with the standard of care, methotrexate (MTX). Each was administered with MTX in patients without prior biologic DMARD therapy. The 1-year results of this blinded 2-year study showed comparable efficacy, radiographic progression, and safety for patients treated with ABA versus ADA [Weinblatt ME et al. Arthritis Rheum 2013].
The objective of the AMPLE Phase 3b trial, presented by Michael Schiff, MD, University of Colorado, Denver, Colorado, USA, was to compare the efficacy and safety of ABA versus ADA after 2 years of treatment in biologic DMARD-naïve patients with RA. A total of 646 patients were randomized to treatment with subcutaneous (SC) ABA 125 mg weekly plus MTX (n=318) or SC ADA 40 mg biweekly plus MTX (n=328). All patients had active RA for ≤5 years and an inadequate response to MTX. The primary endpoint was the American College of Rheumatology 20% improvement criteria (ACR20) response at 1 year. The secondary endpoints were injection-site reactions at 1 year and ACR responses, 28-joint disease activity score (DAS28) based on C-reactive protein (CRP), and radiographic inhibition, safety, and retention at 2 years.
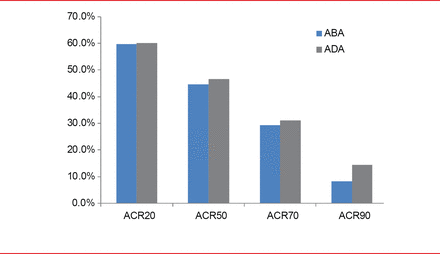
After 2 years, ACR responses with ABA versus ADA were as follows: ACR20, 59.7% versus 60.1%; ACR50, 44.7% versus 46.6%; ACR70, 29.3% versus 31.1%; ACR90, 8.2% versus 14.5% (Figure 1).
ACR Response Through 2 Years
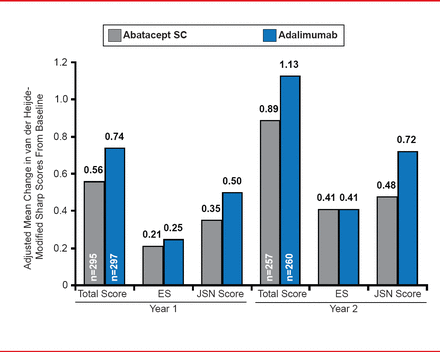
The adjusted mean change in DAS28-CRP at 2 years was <2.6 in both treatment groups. Radiographic outcomes showed an adjusted mean change in the modified total Sharp score (mTSS) of 0.89 with ABA treatment versus 1.13 with ADA therapy (Figure 2). The adjusted mean change in erosion score was 0.41 in both treatment groups and the adjusted mean change in joint space narrowing was 0.48 with ABA versus 0.72 with ADA. The differences in radiographic outcomes between the two groups were not statistically significant. The cumulative probability of change in mTSS from baseline to Year 2 was 84.8% in the ABA arm compared with 83.8% in the ADA arm.
Radiographic Outcomes at Years 1 and 2
ES=echographic score; JSN=joint-space narrowing.
Reproduced with permission from M Schiff, MD.
Adverse event (AE) rates were similar in both treatment groups, at 92.8% in the ABA arm and 91.5% in the ADA arm (Table 1). Serious AE (SAE) rates were 13.8% with ABA and 16.5% with ADA. Serious infections were reported in 3.8% of ABA and 5.8% of ADA patients. AEs leading to discontinuation occurred in 3.8% of patients treated with ABA and 9.5% of those treated with ADA. Two cases of tuberculosis were seen in Year 2 in the ADA+MTX arm.
Adverse Events Through 2 Years of Treatment
The AMPLE trial was the first study comparing two biologic agents in patients with RA who had an inadequate response to MTX. The 2-year efficacy results were similar to those reported at 1 year, with comparable responses in both treatment groups. The overall safety outcomes were similar in both groups but fewer patients treated with ABA discontinued treatment due to AEs, SAEs, and serious infections. The outcomes of the AMPLE trial demonstrate the comparable efficacy of ABA and ADA, and provide clinicians with evidence supporting the use of either biologic for the treatment of RA in patients with an inadequate response to MTX.
- © 2013 MD Conference Express®