Summary
An investigational selective inhibitor of Janus kinase 1 (JAK1) was found to improve clinical endpoints with a favorable safety profile in a Phase 2a dose-ranging study of patients with rheumatoid arthritis (RA). This article discusses the results of a 4-week double-blind, placebo-controlled study of GLPG0634, an orally-available, selective inhibitor of JAK1, in patients with active RA [Vanhoutte FP et al. EULAR 2013 (poster 0229)].
- Rheumatoid Arthritis
- Rheumatology
- Rheumatoid Arthritis
An investigational selective inhibitor of Janus kinase 1 (JAK1) was found to improve clinical endpoints with a favorable safety profile in a Phase 2a dose-ranging study of patients with rheumatoid arthritis (RA).
Frédéric P. Vanhoutte, MD, Galapagos NV, Mechelen, Belgium, presented the results of a 4-week double-blind, placebo-controlled study of GLPG0634, an orally-available, selective inhibitor of JAK1, in patients with active RA [Vanhoutte FP et al. EULAR 2013 (poster 0229)]. JAKs are critical components of signaling mechanisms used by a number of cytokines and growth factors, including those that are elevated in patients with RA. GLPG0634 had demonstrated encouraging efficacy and safety in a 4-week proof-of-concept trial in RA.
The current study was conducted in 91 patients with active RA who had an insufficient response to methotrexate (MTX) and were naïve to biological agents. They were randomized to GLPG0634 at doses of 30, 75, 150, and 300 mg, or placebo, all once daily. All patients continued to take their stable background therapy of MTX.
The mean duration of RA was 4.4 years in the placebo group compared with 7.9 to 10.0 years in the active treatment groups. Mean scores on the disability activity score in 28 joints (DAS28) C-reactive protein (CRP) at baseline ranged from 5.7 to 6.4. The group randomized to 150 mg of GLPG0634 had worse disease at baseline than the other groups, as evident by the longest duration of RA (10 years), the highest DAS28 score (6.4), and the highest scores (worst) on the Health Assessment Questionnaire-Disability Index (HAQ-DI), tender joint count (TJC), and swollen joint count (SJC).
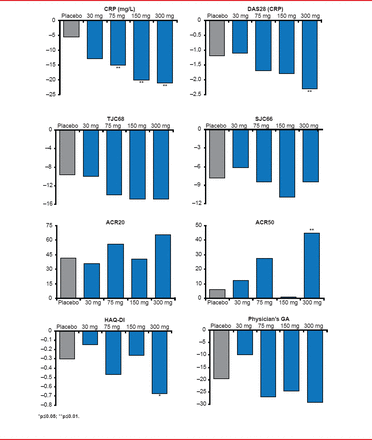
Dose-dependent improvements in CRP, DAS28-CRP, American College of Rheumatology 20% improvement criteria (ACR20), ACR50, TJC, SJC, and HAQ-DI were found for GLPG0634, with little improvement at the 30-mg dose and the best results achieved with the 300-mg dose. Relative to placebo, statistically significant improvements were found with GLPG0634 on CRP, DAS28, ACR50, and HAQ-DI.
Several efficacy endpoints showed statistical significance in favor of GLPG0634 (Figure 1). The reduction in CRP level from baseline ranged from 15 to 21 mg/L (p<0.01 for all 3 doses vs placebo). All GLPG0634 dose levels also showed improvement in DAS28 (−1.7 to −2.3; p≤0.01 at 300 mg) and in HAQ-DI (−0.47 to −0.57; p≤0.05 at 300 mg). This was not the case for achieving an ACR20 response (41% of patients on placebo vs 65% on 300 mg), but it was for ACR50 (6% for placebo vs 45% on 300 mg).
Results of the Efficacy Endpoints
ACR20=American College of Rheumatology 20% improvement criteria; CRP=C-reactive protein; DAS28=disability activity score in 28 joints; GA=general assessment; HAQ-DI=Health Assessment Questionnaire-Disability Index; SJC=swollen joint count; TJC=tender joint count.
Reproduced with permission from FP Vanhoutte, MD.
The overall safety profile of GLPG0634 was favorable (Table 1). GLPG0634 was associated with a limited decrease in absolute neutrophil count, no neutropenia, and no impact on lymphocyte differentials. No increases in levels of low-density lipoprotein cholesterol and no elevations in liver transaminases (alanine aminotransferase/aspartate transaminase) were observed. A modest improvement in hemoglobin was induced by GLPG0634.
Most adverse events were mild and no serious adverse events were reported. No patient discontinued due to adverse events.
These early clinical results demonstrated that selective inhibition of JAK1 by once-daily dosing of GLPG0634 from 75 to 300 mg was efficacious and generally well tolerated for 4 weeks' treatment of RA, concluded Dr. Vanhoutte. Larger, longer-term studies in RA are being initiated to evaluate optimal doses for efficacy and safety.
- © 2013 MD Conference Express®