Summary
While progress has been made in predicting response to therapy for juvenile rheumatoid arthritis, major efforts are still needed in some areas before individualized treatment will be possible. This articles explores the current status of this topic for juvenile idiopathic arthritis, biomarker development, and cytokine blockade.
- Inflammatory Disorders
- Arthritis
- Inflammatory Disorders
- Rheumatology
- Arthritis
While progress has been made in predicting response to therapy for juvenile rheumatoid arthritis (JRA), major efforts are still needed in some areas before individualized treatment will be possible. This session explored the current status of this topic for juvenile idiopathic arthritis (JIA), biomarker development, and cytokine blockade.
PREDICTION OF RESPONSE TO THERAPY: LESSONS FROM JIA
Nico Wulffraat, MD, PhD, University Medical Center, Utrecht, The Netherlands, spoke about the prediction of response to therapy for patients with JIA. Risk factors for poor treatment outcomes include female sex, polyarticular and symmetrical joint involvement, elevated inflammatory markers, and rheumatoid factor (RF) positivity [Adib N et al. Rheumatology (Oxford) 2005]. However, that research was conducted prior to the impact of treating JIA with biologic drugs, said Prof. Wulffraat.
Another study suggested that the persistent oligoarticular JIA (pers-OA-JIA) subtype of JIA predicted a relatively benign, self-remitting path, while extended OA JIA (ext-OA-JIA) fared less favorably [de Kleer IM et al. J Immunol 2004]. Peripheral blood of patients with pers-OA-JIA had a significantly higher frequency of CD4+CD25bright regulatory T cells and higher levels of mRNA FOXP3 than ext-OA-JIA patients. It may be that these regulatory T cells mature in the synovial fluids of the joint.
Other research found that starting treatment (methotrexate [MTX]) earlier after diagnosis can improve response at 6 months [Albers HM et al. Arthritis Rheum 2009] and that young patients (>3 years 6 months) who start treatment early with etanercept and do not have wrist involvement are more likely to achieve inactive disease status [Solari N et al. J Rheumatol 2013].
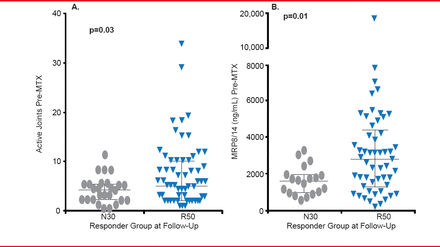
Clinical measures taken soon after diagnosis may help with prediction of treatment response. A Canadian study found that the Juvenile Arthritis Quality of Life Questionnaire is a predictor of multiple outcomes [Oen K et al. Arthritis Rheum 2009]. A very recent study found that high levels of baseline MRP8/14 (>3000 ng/mL) predicted the subgroup of patients more likely to respond to MTX (Figure 1) [Moncrieffe H et al. Rheumatology (Oxford) 2013].
Genetics research has revealed polymorphisms associated with a positive clinical response to MTX and produced a model to predict outcome. One study showed a positive association with polymorphisms in the AMPD1, ATIC, and ITPA genes [Wessels JA et al. Arthritis Rheum 2006]. In other work, Prof. Wulffraat and colleagues described a model they developed and validated that can identify nonresponders through clinical and genetic variables [Bulatovic M et al. Ann Rheum Dis 2012].
Myeloid-Related Protein May Predict Response
MTX=methotrexate.
Reproduced from Moncrieffe H et al. A subgroup of juvenile idiopathic arthritis patients who respond well to methotrexate are identified by the serum biomarker MRP8/14 protein. Rheumatology (Oxford) 2013; 52(8):1467–1476. With permission from Oxford University Press.
While prediction of response to therapy largely relies on subtype, disease duration, and disease activity, increasing biomarker development activity will hopefully improve individualized medicine, Prof. Wulffraat concluded.
BIOMARKERS PREDICTING RELAPSES IN INFLAMMATORY ARTHRITIS
Dirk Foell, MD, University of Münster, Münster, Germany, expanded on the topic of biomarkers. Although drug-free remission can be achieved in 17% to 29% of patients and sustained in 9% to 16% during a 1- to 4-year period, low disease activity can be reached again in most patients who restart treatment [van den Broek M et al. Curr Opin Rheumatol 2011]. However, patients with JIA often experience inactive and active episodes of disease over a course of 5 years [Wallace CA et al. Arthritis Rheum 2005].
Power Doppler ultrasound has proven beneficial in predicting relapse and remission responses. A recent study found that ultrasound-detected abnormalities may be common in children with JIA in remission, but it does not predict early flare [Magni-Manzoni S et al. Ann Rheum Dis 2012]. An earlier study found that for patients with RA in remission or with low disease activity, ultrasound could predict relapse or radiographic progression and predict adequately controlled disease in patients [Foltz V et al. Arthritis Rheum 2012].
To better reach the goal of remission, Prof. Foell said, researchers need to define remission, which has been subject to ever-changing criteria [van den Broek M et al. Curr Opin Rheumatol 2011]. While defining remission is based mainly on clinical manifestations of disease, additional criteria have been added since the preliminary definition of disease improvement in 1997 by the American College of Rheumatology [Ringold S, Wallace CA. Curr Opin Rheumatol 2007]. Biomarkers, such as erythrocyte sedimentation rate and C-reactive protein (CRP) have been included for a long time.
More studies have focused on biomarkers. One study identified MRP8/14 complexes as new inflammatory components that activate phagocytes for protective effects [Vogl T et al. Nat Med 2007]. Another study showed that normal serum levels of MRP8/14 in patients with clinically inactive JIA may predict that MTX treatment can be safely withdrawn after remission [Foell D et al. Ann Rheum Dis 2004].
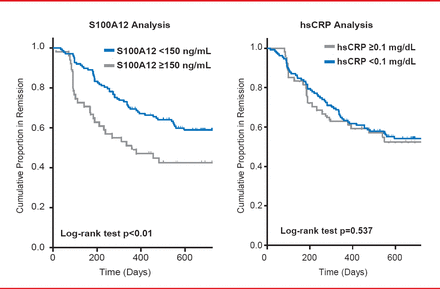
Another study suggests a possible role for S100A12 in synovitis and points to high serum concentrations of the same protein as a useful serum marker in patients with active arthritis in remission (Figure 2) [Foell D et al. Rheumatology (Oxford) 2003]. A recent study demonstrated that a combination of S100A12 and high-sensitivity CRP has at present the best predictive power for the risk of a disease relapse out of a status of instable clinical remission [Gerss J et al. Ann Rheum Dis 2012]. A strategy for the stratification of patients for maintenance therapy versus withdrawal of anti-inflammatory drugs in times of disease remission has been proposed and a prospective trial applying this strategy for personalized medicine is under way.
Biomarkers for the Risk of Relapse After Stopping Therapy
hsCRP=high-sensitivity C=reactive protein.
Reproduced with permission from D Foell, MD.
Remission can be reached by children with JIA and adults with RA. The full potential of imaging modalities and biomarkers has not been defined. Yet, S100 biomarkers indicate subclinical inflammation, which may help to identify patients with increased risk of flares and allow stratification of patients for therapeutic decision-making.
NEW STRATEGIES TO PREDICT RESPONSE TO CYTOKINE BLOCKADE
Georg Schett, MD, University of Erlangen-Nuremberg, Erlangen, Germany, reviewed the need for better understanding cytokines and tumor necrosis factor (TNF) blockers. A TNF inhibitor (TNFi) can achieve a moderate to good response (EULAR criteria) in about half of RA patients, but to date no reliable predictor of treatment response has been identified. Blocking TNF, which induces hyperalgesia associated with higher neuronal activity in central nervous system areas involved in pain perception, can block activation of the pain pathway in mice and humans.
The most widely used tool for detecting brain activity is functional magnetic resonance iomaging (fMRI). Schett and colleagues demonstrated on fMRI that blocking TNF-α produced a positive response in the thalamus and somatsensoric cortex within 24 hours of administration of a monoclonal antibody to TNF-α [Hess A et al. Proc Natl Acad Sci USA 2011]. Their results also showed that the response in the brain preceded any response in the joints, which suggests that joint compression also induces activity in the limbic system, relevant for inner body sensation.
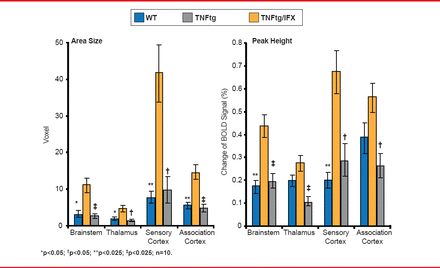
Schett and colleagues then used fMRI to assess activity in the brain and hand and to conduct a clinical assessment in 10 patients with RA before and 3, 7, and 28 days after the start of TNFi treatment. While responders showed significantly higher activity in the thalamic, limbic, and associated brain areas (Figure 3), brain activity decreased within 3 days after exposure, which preceded clinical responses on Day 7 and MRI-observed hand responses on Day 28.
The PRECAPRA trial will evaluate whether high fMRI activity can predict response to therapy with a TNFi. The Phase 3 multicenter, randomized controlled trial will compare certolizumab and placebo in 52 patients.
TNF Blockade Affects Normal Brain Activity
BOLD=blood-oxygen level-dependent; IFX=infliximab; TNFtg=tumor necrosis factor transgenic; WT=wild-type.
Reproduced with permission from G Schett, MD.
- © 2013 MD Conference Express®