Summary
Patients with a ruptured thoracic aortic aneursym experience among the highest rates of mortality reported for any cardiovascular condition. When repair is performed before rupture, these rates can be reduced from 48% to >19% at 30 days, and from 62% to >31% through 365 days. In both situations, however, the increasing mortality after 30 days demonstrates a significant risk that extends beyond the initial perioperative period. This article discusses the techniques used to minimize the risk of endoleaks in patients being treated for aortic aneurysms with endovascular techniques.
- Interventional Radiology
- Interventional Techniques & Devices
- Interventional Radiology
- Interventional Techniques & Devices
- Cardiology
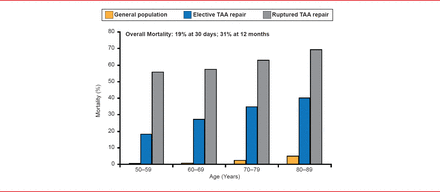
Patients with a ruptured thoracic aortic aneursym experience among the highest rates of mortality reported for any cardiovascular condition. When repair is performed before rupture, these rates can be reduced from 48% to >19% at 30 days, and from 62% to >31% through 365 days. In both situations, however, the increasing mortality after 30 days demonstrates a significant risk that extends beyond the initial perioperative period. This risk increases with age (Figure 1) [Rigberg DA et al. J Vasc Surg 2006].
Impact of Age on Mortality for Patients Undergoing TAA Repair
TAA=thoracoabdominal aneurysms.
Reproduced from Rigberg DA et al. Thirty-day mortality statistics underestimate the risk of repair of thoracoabdominal aortic aneurysms: A statewide experience. J Vasc Surg 2006;43(2):217–222. With permission from Elsevier.
Approximately 30% of patients being evaluated for aortic aneurysm repair are considered at too high a risk for surgery. Endovascular aortic repair-thoracic endovascular aortic/aneurysm repair (EVAR-TEVAR) is increasingly being used in this high-risk population. Achieving procedural success depends on multiple factors but the deploying of the device so as to minimize the potential for endoleaks (leakage off blood from the graph into the aneurysm sac) is critical to achieving procedural success.
Oscar A. Mendiz, MD, Hospital Universitario, Fundación Favaloro, Buenos Aires, Argentina, discussed the techniques used to minimize the risk of endoleaks in patients being treated for aortic aneurysms with endovascular techniques. Individual variations in vascular anatomy that make EVAR-TEVAR challenging include aneurysms with a short neck (<15 mm for abdominal aneurysms, <20 mm for thoracic aneurysms), angulated aneurysm necks (>40°), mural thrombus in the neck, tapering or reverse tapering of the aneurysm sac, compromise of both iliac arteries, thoracoabdominal aneurysms, and juxtarenal aneurysms.
Despite increases in the number of endograft devices and the improved design, many patients do not meet the indications for the current Food and Drug Administration-approved devices. Although custom devices are available, these devices take considerable time to acquire. As a result, surgeons often create or modify grafts to address patients anatomical variation. Stents are deployed along with the endograft, and the resulting “chimneys” allow an adequate proximal landing zone while enabling blood flow of the branch vessels outside of the endograft. Myriad versions of the chimney technique can be employed as dictated by the anatomy; these can be a cheaper and faster solution, and seem to have acceptable outcomes.
Abdominal aortic aneurysm (AAA) often occurs in the area of the renal arteries. In the past, this has prevented the use of a stent graft since the graft itself would cover the renal arteries preventing blood flow to the kidneys. Recently, fenestrated stent grafts have been developed which have small holes in the stent graft and allow blood to flow to the important organs. Although it is a challenging procedure, successful results have been achieved using fenestrated endovascular aneurysm repair (FEVAR).
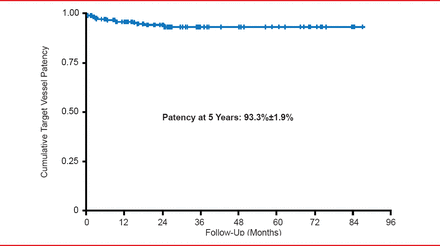
In a meta-analysis of 11 studies describing the use of FEVAR for suprarenal and juxtarenal AAAs (660 procedures), 11 deaths occurred within 30 days, yielding a 30-day proportional mortality rate of 2%. Target vessel perfusion rates ranged from 90.5% to 100% [Cross J et al. Br J Surg 2012]. Intermediate results from another study using FEVAR for juxtarenal aortic aneurysms with short proximal necks, reported no aneurysm-related deaths, ruptures, or conversions through 2 years of follow-up. No type I or II endoleaks were reported. Type II endoleaks were noted in 26.1% of patients at 12 months and 20.0% of patients at 24 months [Greenberg RK et al. J Vasc Surg 2009]. In an 8-year clinical study, cumulative visceral branch patency was 93.3±1.9% at 5 years. All visceral artery stent occlusions occurred within the first 2 postoperative years. Renal function deterioration, however, was a major concern (Figure 2) [Verhoeven EL et al. Eur J Vasc Endovasc Surg 2010].
Target Vessel Patency With the FEVAR Approach
Reproduced from Verhoeven EL et al. Fenestrated Stent Grafting for Short-necked and Juxtarenal Abdominal Aortic Aneurysm: An 8-Year Single-centre Experience. Eur J Vasc Endovasc Surg 2010;39(5):529–536. With permission from Elsevier.
About one third of patients with AAAs have anatomy that is unfavorable to the use of a stent graft [Hallet JW. Comprehensive Vascular and Endovascular Surgery. Edinburgh, Scotland; New York, NY: Mosby; 2004]. These patients can still be treated successfully with endovascular aneurysm repair [Stather PW et al. Eur J Vasc Endovasc Surg 2012] with the right selection of fenestrated endografts and proper surveillance. The design must be individualized using precise imaging of the patient's AAA. Fenestrated endovascular repair of AAA has been proposed as an alternative to open surgery for juxtarenal and pararenal AAAs. The Global Collaborators on Advanced Stent-Graft Techniques for Aneurysm Repair [GLOBALSTAR] Registry is the largest cohort of patients with fenestrated endovascular repair of juxtarenal aortic aneurysms in the United Kingdom. Data from 318 patients were obtained from 14 centers. Primary procedural success was achieved in 99% of patients, perioperative mortality was 4.1%, and intraoperative target vessel loss was observed in 5 of 889 target vessels (0.6%). Freedom from reintervention at 1 year was 90%. After 3 years, the survival rate was 89% [British Society for Endovascular Therapy and the GLOBALSTAR Registry. Circulation 2012]. The use of fenestrated endografts for aortic aneurysm repair is safe and effective in preventing rupture in the medium term, but there is a predictable high mortality rate during follow-up in this high-risk cohort, which requires meticulous follow-up [Amiot S et al. Eur J Vasc Endovasc Surg 2010].
The chimney graft has evolved as a potential alternative to fenestrated and side-branched endografts in high-risk patients with juxtarenal, pararenal, or thoracoabdominal aneurysms. Primary technical success was achieved in all patients in one meta-analysis of 15 reports. The 30-day in-hospital mortality was 4.3%. Although this technique has achieved relatively good results, long-term endograft durability and proximal fixation remains a significant concern [Moulakakis KG et al. J Vasc Surg 2012]. The technique should be considered only in acute poor surgical risk patients, as a bailout in case of unintentional renal artery coverage, or in elective poor surgical risk cases that are not suitable for a fenestrated endograft [Karsargyris A et al. J Endovasc Ther 2013]. This approach will require further investigation before widespread adoption.
An approach using coils and external-internal iliac bypass has been used in cases of bilateral iliac aneurysms when both iliac arteries are compromised. In a long-term study of iliac aneurysm repair with an iliac-branched endograft, periprocedural technical success rate was 95% with no mortality. Estimated patency rate of internal iliac branches after 5 years was 91.4%. Freedom from any reintervention was 90% at 1 year and 81.4% at 5 years. No late ruptures occurred and there was a low risk of reintervention. This technique can be considered as a first endovascular option in patients with extensive iliac aneurysm disease and favorable anatomy [Parlani G et al. Eur J Vasc Endovasc Surg 2012]. Results comparing side-branch endograft deployment with hypogastric exclusion for endovascular treatment of iliac aneurysms showed no significant differences in the failure of hypogastric side branch deployment (2/32) compared with hypogastric coiling (3/42). Reintervention rates were also similar (5/32 vs 4/42) at 1 year. Buttock claudication or impotence was more frequent after hypogastric exclusion, however [Verzini F et al. J Vasc Surg 2009].
- © 2013 MD Conference Express®